Rep:Mod:VN3944
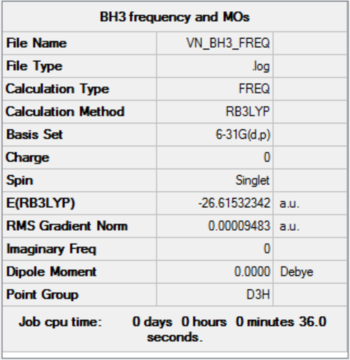
BH3
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000190 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000747 0.001800 YES RMS Displacement 0.000374 0.001200 YES
Optimisation log file: logfile
Frequency analysis log file: logfile
Low frequencies --- -0.2260 -0.1035 -0.0054 48.0278 49.0875 49.0880 Low frequencies --- 1163.7224 1213.6715 1213.6741
Optimised BH3 |
IR analysis

| Freq (cm-1 | Mode | IR active? | Intensity |
|---|---|---|---|
| 1163 | Wag | YES | Strong |
| 1213 | Bend | YES | Weak |
| 1213 | Bend | YES | Weak |
| 2579 | Symm Stretch | NO | No peak |
| 2712 | Asymm Stretch | YES | Strong |
| 2712 | Asymm Stretch | YES | Strong |
Only three peaks can be observed in the IR because two of the vibrational modes are degenerate in energy (1213 and 2712 cm-1) and the symmetric stretch at 2579 cm-1 is not IR active.
Ng611 (talk) 17:47, 17 May 2018 (BST) Good analysis -- remember to report the intensities of the various modes too
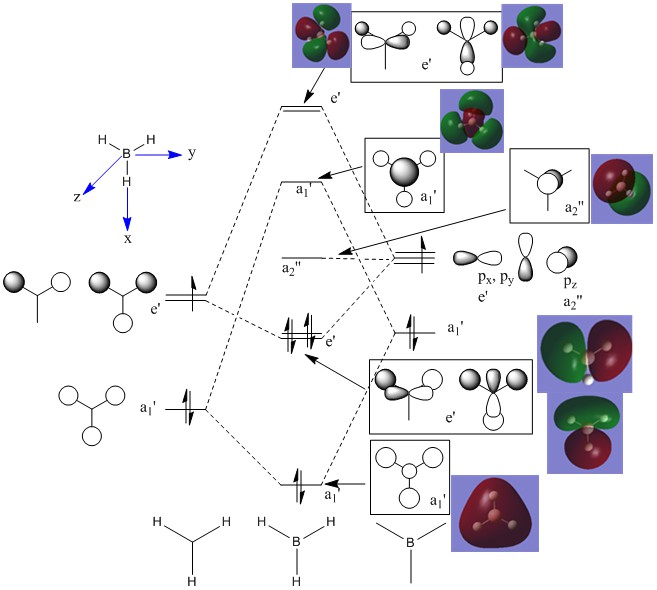
MO diagram
Ng611 (talk) 17:49, 17 May 2018 (BST) Good MO analysis. From comparing the calculated and qualitative MOs, are there any differences at all between them, and what does this tell you about qualitative MO theory?
Association Energy: ammonia-borane
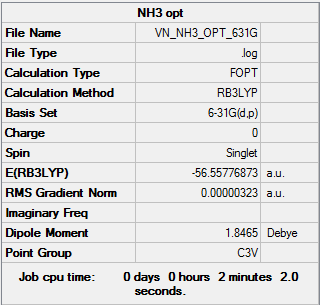
NH3
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Optimisation output file: File
Frequency analysis: File
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
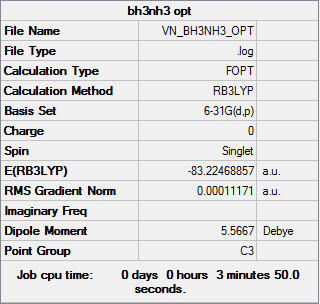
BH3-NH3
Item Value Threshold Converged? Maximum Force 0.000233 0.000450 YES RMS Force 0.000083 0.000300 YES Maximum Displacement 0.000981 0.001800 YES RMS Displacement 0.000370 0.001200 YES
Output of optimisaton: File
Frequency analysis: File
Low frequencies --- -0.1144 -0.1063 -0.0333 15.1196 15.2002 38.3644 Low frequencies --- 265.8918 634.5284 639.3073
Calculation of Association Energy
E(NH3)= -56.55777 a.u. E(BH3)= -26.61532 a.u. E(NH3BH3)= -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -0.0516 a.u. = -135.47 kJ/mol
The dissociation energy will therefore be 135.47 kJ/mol.
Based on these calculations, the Nitrogen-Boron dative bond can be considered quite strong. A C-C bond, in fact, has a dissociation energy of about 154 kJ/mol.
Ng611 (talk) 19:38, 15 May 2018 (BST) Remember to round your figures to the nearest Kj/mol and to include specific literature references for any numbers you provide.
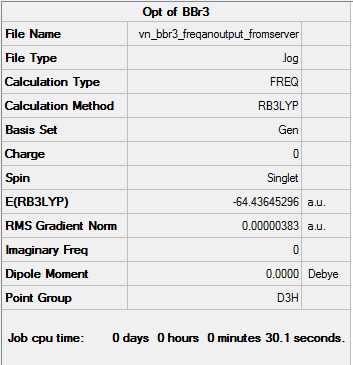
BBr3
The optimisation and the frequency analysis were both carried out using the SCAN server. DOI:10.14469/ch/198191 Link of the file on DSpace: click here
Optimisation logfile: logfile
Frequency analysis log file: logfile
The basis set used for B was 6-31G(d,p) and for Br(LanL2DZ).
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Main group halides
Isomers
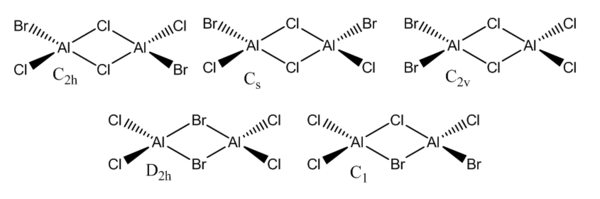
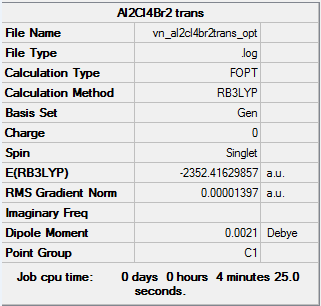
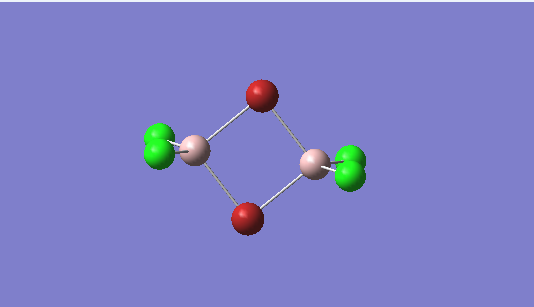
The C2h and the D2h isomers were analysed. The former was found to be the lowest energy isomer, as it can be seen in the summary tables below.
 |
|
 |
|
The basis set used for Al and Cl was 6-31G(d,p) and for Br(LanL2DZ)
Logfile of first isomer: log
Logfile of second isomer: log
Item table of first isomer:
Item Value Threshold Converged? Maximum Force 0.000039 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.001573 0.001800 YES RMS Displacement 0.000748 0.001200 YES
Item table of second isomer:
Item Value Threshold Converged? Maximum Force 0.000026 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.001036 0.001800 YES RMS Displacement 0.000354 0.001200 YES
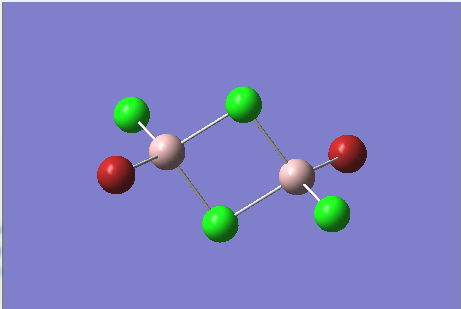
Optimised First Isomer |
The energy of the first isomer is -6176269.44 kJ/mol, whereas for the second isomer it is -6176243.26 kJ/mol.

Logfile of the monomer: log
Item table of the monomer:
Item Value Threshold Converged? Maximum Force 0.000040 0.000450 YES RMS Force 0.000025 0.000300 YES Maximum Displacement 0.000283 0.001800 YES RMS Displacement 0.000165 0.001200 YES
Relative stability
The isomer with two bridging Cl atoms is more stable than the isomer with Br bridging atoms because there is a better overlap in the three-center bond, and less steric hindrance.
Ng611 (talk) 19:41, 15 May 2018 (BST) A calculation demonstrating the change in relative energies of these two molecules would have improved this section significantly.
Dissociation energy
The monomer has an energy of -1176.19012 a.u. and the dissociation energy of the first isomer can be calculated with the equation:
ΔE=E(Al2Cl4Br2 (trans))-[2*E(AlCl2Br)]=-6176269.44 - [2(-3088087.42)]= -94.6 kJ/mol
The dissociation energy will therefore be 94.6 kJ/mol. The product is found to be more stable than the single monomers and this is the force that drives the association of the monomers.
Ng611 (talk) 19:42, 15 May 2018 (BST) Remember to round your results!
Molecular orbitals and LCAO
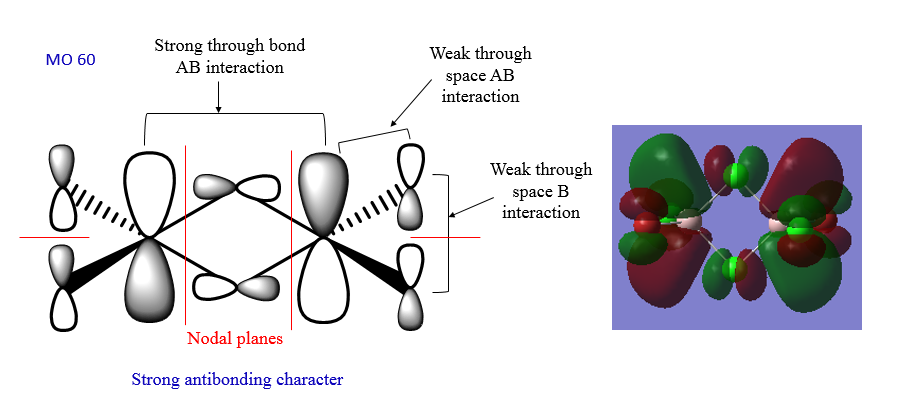
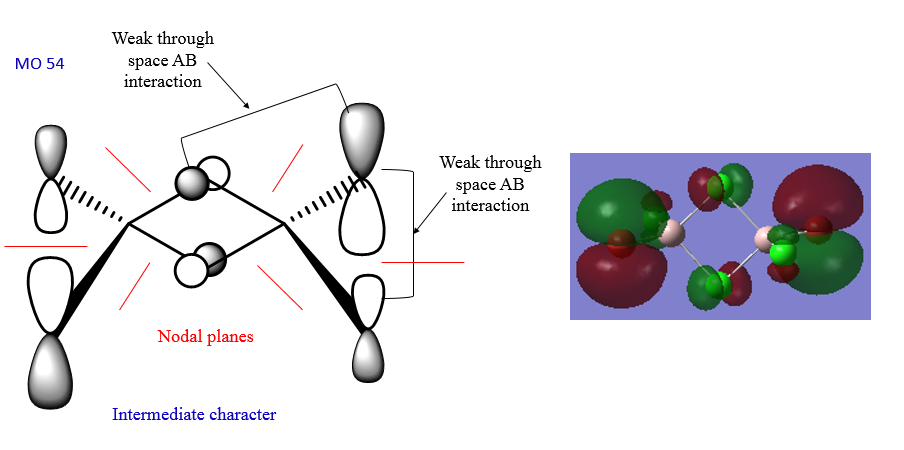
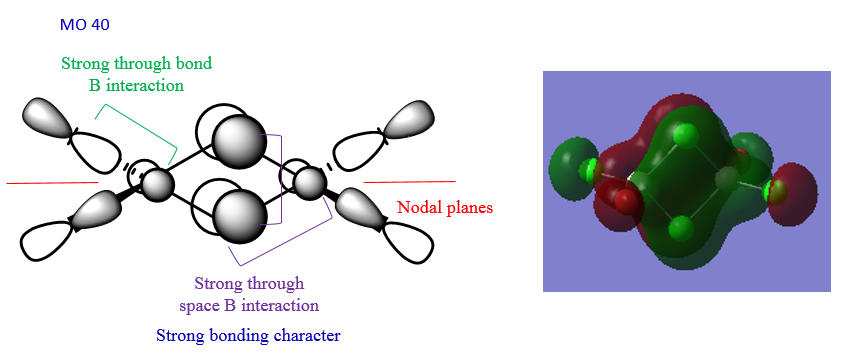
The MOs of the lowest energy isomer were visualised. The LCAO analysis of three of these MOs can be seen below.
|
|
|
In MO40 there is a good overlap between the p orbitals on Cl and Br and the p orbitals of the Al atoms. Also, there is a good p-p overlap between the Al atoms and the bridging Cl atoms. MO54 has an intermediate character because the thrgough-space interactions are not strong enough to make it a full antibonding orbital. The overlap is not good. MO60 has a strong antibonding character because there is a strong thorugh-bond interaction between the p orbitals on the bridging Cl atoms and the Al atoms, and also a quite strong antibonding interaction between the p orbitals on the terminal groups and the Al p orbitals.
Ng611 (talk) 19:49, 15 May 2018 (BST) A good analysis here. For your second entry in the table, describing an orbital as being of 'intermediate' character doesn't tell us anything. Other LCAO decompositions and analyses were good though.
Ng611 (talk) 19:49, 15 May 2018 (BST) Overall a good report. You missed out a number of marks by reporting figures to too high an accuracy value (remember, DFT results are only accurate to about 1kJ/mol, and potentially even less accurate than that). YOur section on LCAO and bonding was very good.