Rep:Mod:VMS1315
Computational Lab - Intro. to molecular modelling 2 (Varun Mann)
NH3 Molecule
Part 1
What is the molecule? NH3 (Ammonia)
What is the calculation method? RB3LYP
What is the basis set? 6-31G(d,p)
Final energy E(RB3LYP) is -56.55776873 a.u.
RMS Gradient is 0.00000485 a.u.
Point group of molecule - C3V
N-H Bond length = 1.01798 Å
H-N-H Bond angle = 105.741°
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Ammonia |
Part 2
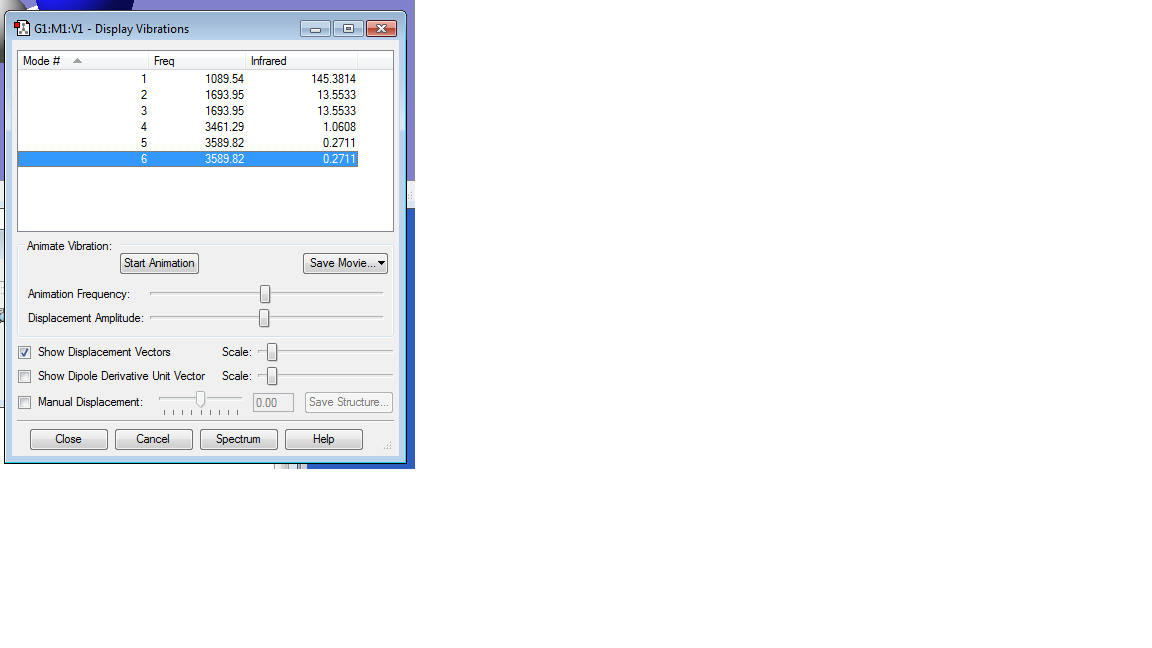
How many modes do you expect from the 3N-6 Rule? 3
Which modes are degenerate (ie have the same energy)? 2,3 and 5,6
Which modes are "bending" vibrations and which are "bond stretch" vibrations? 1,2,3 are bends and 4,5,6 are stretches
Which mode is highly symmetric? Mode 4
One mode is known as the "umbrella" mode, which one is this? Mode 1
How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2
N atom charge = -1.125
H atom charge = 0.375
I would expect the charge on the N atom to be negative and the charge on the H atom to be positive because N is more electronegative than H.
Part 3
N2
What is the molecule? N2 (Nitrogen)
What is the calculation method? RB3LYP
What is the basis set? 6-31G(d,p)
Final energy E(RB3LYP) is -109.52412868 a.u.
RMS Gradient is 0.00000060 a.u.
Point group of molecule - D(infinity)h
N-N(triple bond) Bond length = 1.10550 Å
N-N(triple bond) bond angle = 180°
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Vibrational frequencies for N2: 2457.33
H2
What is the molecule? H2 (Hydrogen)
What is the calculation method? RB3LYP
What is the basis set? 6-31G(d,p)
Final energy E(RB3LYP) is -1.17853936 a.u.
RMS Gradient is 0.00000017 a.u.
Point group of molecule - D(infinity)h
H-H Bond length = 0.74279 Å
H-H bond angle = 180°
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Vibrational frequency for H2: 4465.68
Calculations
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -113.1155375 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = -0.05579074 a.u.
ΔE=-146.4785879 kJ/mol
Ammonia product is more stable
Part 4/5
My own molecule: O2
What is the molecule? O2
What is the calculation method? RB3LYP
What is the basis set? 6-31G(d,p)
Final energy E(RB3LYP) is -150.25742434 a.u.
RMS Gradient is 0.00007502 a.u.
Point group of molecule - D(infinity)H
O=O Bond length = 1.21602 Å
O=O Bond angle = 180°
Item Value Threshold Converged?
Maximum Force 0.000130 0.000450 YES
RMS Force 0.000130 0.000300 YES
Maximum Displacement 0.000080 0.001800 YES
RMS Displacement 0.000113 0.001200 YES
Vibrational frequency is 1642.74
Charge is 0 on each oxygen atom because O2 is a homonuclear diatomic molecule.
Oxygen |
Molecular orbitals for O2
The 1s orbital of each oxygen atom contributes to this occupied sigma bonding orbital (1σ). This MO contributes little to the overall bonding of the two oxygen atoms because there is very little overlap between the two 1s orbitals. The MO is very deep in energy so will not be involved in the molecule's reactivity.
The 2s orbital of each oxygen atom contributes to this occupied sigma bonding orbital (2σ). There is a large overlap between the two 2s orbitals and so this MO will contribute more to the bonding of the molecule. The energy of this MO is low (although comparatively with the 1σ it is much higher in energy) and so will not contribute much to the molecule's reactivity.
This is the 2σ* occupied anti-bonding orbital. It is higher in energy than the 2σ bonding orbital.
This is the 1π occupied bonding orbital. This is formed by overlap of the 2p orbital of each oxygen atom. As the overlap is significant the MO will contribute a significant amount to the bonding of the molecule. The MO is relatively high in energy compared to the other MOs considered.
This is a 1π* occupied anti-bonding orbital. This anti-bonding MO's energy is in the HOMO region and therefore is directly involved in the reactivity of the molecule with other species.
Independent work
CH4
What is the molecule? CH4
What is the calculation method? RB3LYP
What is the basis set? 6-31G(d,p)
Final energy E(RB3LYP) is -40.52275298 a.u.
RMS Gradient is 0.00803769 a.u.
Point group of molecule - T
C-H Bond length = 1.07 Å
H-C-H Bond angle = 109.5°
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Methane |