Rep:Mod:TomTaylorMod3
The Transition State
The Cope Rearrangement
A example of the Cope Rearrangement is shown below:
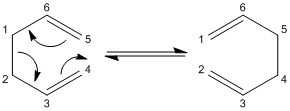
This reaction goes through either a chair cyclohexane like transition state or a boat cyclohexane like transition state. It will be investigated which of these two possible transition states the reaction goes though. But first, the reactant/product of this reaction, 1,5 hexadiene will be studied in order to find it minimum energy confirmation.
Confirmations of 1,5 Hexadiene
The different confirmations of 1,5 Hexadiene were investigated in order to find its lowest energy confirmations. In general, it can be thought that 1,5 Headiene can have one of two types of confirmations when looking down the carbon 3 and carbon 4 bond; Gauche or Anti. This is shown below:
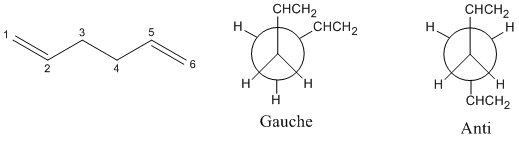
With in these two types of comfirmations, a number of different comfirmations are possible depending on the orination of the -CHCH2 groups. A number of different Gauche and Anti confirmations were made and opitimised to find their lowest energy comfirmation and the energy of this comfirmation. The find the molecules lowest energy comfirmation, the moleclues were optimised using the Hartree-Fock calculation method and the 3-21G basic state. The type of calculation method used determines the approximations used when solving for the energy using the Schodinger equation and type of basic sets determines the complexcity of the orbitals to used to approximate the real orbitals when solving the Schodinger equation. The results and structures are shown below:
The names under the confirmation heading releate to the structure seen in Appendix 1 on the Moducle 3 wiki site.
File:Gauche1 mol.molFile:Gauche3 mol.molFile:Gauche4 mol.molFile:Anti1 mol.molFile:Anti2 mol.molFile:Anti4 mol.mol
It can be seen that the lowest energy comfirmation of 1,5 Hexadiene is a Gauche comfirmation (Gauche 3). This is an unexpected result as Gauche comfirmations are normally higher in energy conpared to Anti Comfirmations due to the steric interactions between the two larger groups, which are next to each other in a Gauche comfirmation and opposite each other in the Anti comfirmation. However, this result can be explained by looking at the Highest Occupied Molecular Orbitals (HOMO) on the Gauche 3 comfirmation, which is shown below, along with the HOMO for Anti 1 comfirmation for comparison:


It can clearly be seen that in the HOMO of the Gauche 3 comfirmation that there is overlap and interaction between the pi-orbitals of the two alkene units. This lowers the energy of this confirmation and makes it more stable. This decrese in energy is greater than the increase in energy due to steric slashes of the comfirmation (as explainned above, steric slashes occur in Gauche comfirmations) and makes the Gauche 3 comfirmation the most stable. In comparison, it can be seen that there is not pi-orbital overlap between the two alkene units in Anti 1 comfirmation and it is assumed that this is case for all Anti comfirmations due to the orination of the alkene untits, which in Anti comfirmations are far away from each other by virtue of being in a Anti comfirmation (hence few steric slashes compared to the Gauche comfirmations).
It is predicted that the Anti 2 comfirmation of 1,5 hexadiene is the start comfirmations (reactant) when the molecule under goes a Cope rearragement. Therefore, a further optimisation was performed on this comfirmation, using the more complex B3LYP calculation method and the 6-31G d basic set, again more compex than the 3-21G basic set. Therefore this calculation should give results closer to what is seen experimentally. Below is given some structural parameters after the optimisation using the B3LYP method and 6-31G d basic set, as well as the same parameters after the optimsation using the Hartree-Fock method and 3-21G basic set and experimental findings:
| Structural Parameter | Hartree-Fock, 3-21G | B3LYP, 6-31G d | Experimental |
|---|---|---|---|
| C=C Bond length | 1.32Â | 1.33Â | 1.34Â |
| C-C Bond length | 1.50Â | 1.54Â | 1.54Â |
| -C-H Bond length | 1.09Â | 1.10Â | 1.10Â |
| =C-H Bond length | 1.08Â | 1.09Â | 1.09Â |
| H-C-C Angle | 109O | 110O | 110O |
| H-C=C Angle | 122O | 122O | 1200 |
It can be seen that both calculation method/basic sets combinations give structural data that is very similar to that seen experimentally, with the more complex B3LYP/6-31G d calculation/basic set combination giving a slightly better approximation to what is seen experimentally, due to being a more complex calculation method/basic set than the Hartree-Fock/3-21G calculation method/basic set combination.
Calculations can also be perfored to get other data that can be compared to what is seen experimentally. This inculdes the sum of electronic and zero point energies (Electronic energies and vibrational at 0K), sum of electronic and thermal energies (Electronic energies and vibrational at 298K), sum of electronic and thermal enthalpies (sum of electronic and thermal energies with enthalpy correction) and sum of electronic and free energy energies (sum of electronic and thermal energies with entropy correction). These values can be used to give predictions of energy changes for a reaction and can be compared with experiments. These 4 values are shown below, along with total energy, as calculated by the different calculation method and basic sets:
| Energy Term | Hartree-Fock, 3-21G | B3LYP, 6-31G d (298K) | B3LYP, 6-31G d (0.001K) |
|---|---|---|---|
| Sum of Electronic and Zero Point Energies | -231.5395 | -234.4792 | -234.4663 |
| Sum of Electronic and Thermal Energies | -231.5325 | -234.4619 | -234.4663 |
| Sum of Electronic and Thermal Enthalpies | -231.5316 | -234.4609 | -234.4663 |
| Sum of Electronic and Thermal Free Energies | -231.5709 | -234.5001 | -234.4663 |
| Total Energy | -231.6925 | -234.6117 | -234.6095 |
Note, it can be seen from above that for B3LYP, 6-31G d (0.001K) all the energy values are the same (-234.4663 Hatrees) except for the total energy. This is expected as temperature is very close to zero and so the values for the Sum of Electronic and Thermal Energies, the Sum of Electronic and Thermal Enthalpies and the Sum of Electronic and Thermal Free Energies will equal the Sum of Electronic and Zero Point Energies value as the former 3 expressions are exactly the same as Sum of Electronic and Zero Point Energies except they contain different additional terms for thermal energy effects and these will tend to zero as temperture tend to zero.
Optimizing the "Chair" and "Boat" Transition States
As explainned above, the Cope Rearragement can either go though a "Chair" Cyclohexane Transition State (Chair TS) or a "Boat" Cyclohexane Transition State (Boat TS). In order to find out which of the two possible transition states the Cope rearragement processeds by, the energies of the two possible transitions states have to be found and compared. There is a number of ways that the possible transition state can be optimizied in order to find the energy of their lowest energy confirmations and these are deamonstrated below.
The first method is to build a structure that is approximatly like the predicted transitions state and then optimise it's structure as a transition state calculating the force constants in the molecule once, knowing that a transition state has one imagenary frequency (negative frequency on a vibration spectrum). This method was used to find the optimized structure Chair TS. This done by first making an approximate structure of the Chair TS out of two CH2CHCH2 molecules which had been optimised to their lowest energy confrimations using the Hartree-Fock claculation method and the 3-21G basic set of orbitals. These two molecules were positioned to approximate the Chair TS as shown in the Jmol below:
This structure was then optimised, but not to minimum, but to TS (Berny). This was done again using the Hatree-Fock calculation method and the 3-21G basic set. The vibrational spectrum was also calculated using the same method and basic set. I order to decrease the chance of calculation failing and giving an error message, in the additional word section of the calculation set up, "Opt=NoEigen" was typed; this stops the calculation failing if a second negative frequency is calculated for a particular confirmation when the calculation is trying to find the optimized Chair TS structure. The resulting structure for the chair TS is shown below:
Another method similar to the optisation method above, is the "freeze coordinate" method. Again, the approximate structure for the Chair TS as shown above was used, but this time instead of calculating force constants in order to optimise the Chair TS structure to it lowest energy confirmation, the distance between the two pairs of terminal carbons were frozen. The structure was then optimized to its minimum confirmation, using the Hartree-Fock method and the 3-21G basic set. The resulting structure is completly optimiced apart from the two bonds that are forming/breaking. This structure was then used to calculate the structure of the Chair TS by optimising the two bonds that are forming/breaking. This was done by performing a optimisation on the structure to TS (Berny) telling the calculation to derive the bond lengths between the two pairs of terminal carbons. This was done using again the Hartree-Fock method and the 3-21G bacis set. Instead of calculating the force constants in the calculation as was done above in the first method to find the optimised structure of Chair TS, a normal guess Hessian method is used to gain information about the positions of the terimal carbons. The resuling structure of this optimisation for the stucture of Chair TS was the same as the structure shown above. Shown below is a table comparing the results of the two types of optimisations for the lowest energy confirmation of Chair TS:
| Force Constant Method | Freeze Coordinate Method | |
|---|---|---|
| Energy (Hartrees) | -231.6193 | -231.6193 |
| Gradent on the Energy Surface (Hartrees) | 0.000030 | 0.000019 |
| Sum of Electronic and Zero Point Energies (0K) | -231.4667 | -231.4667 |
| Sum of Electronic and Thermal Energies (298K) | -231.4613 | -231.4613 |
It can be seen that both methods give the same energy values and agree with each other, showing bthat both methods are valid and can be used.
The Boat Transition state for the Cope rearragment was found by using a different method called the QST2 method. This involves telling the calculation the reactant and the product for the reaction and then the calculation find the transition point between the two molecules. Below is given the rectant and product for the Cope Rearragement based on the Anti 2 comfirmation of 1,5 Hexadiene.


A QST2 Transition state optimisation calculation was then run, using the Hartree-Fock calculation method and the 3-21G basic set. The resulting transition state structure is shown below:
It can clearly be seen that this transition state optimisation has failed to give the desired Boat transition state. Therefore the QST2 optimisation calculation was tried again using the Hartree-Fock calculation method and the 3-21G basic set again, but this time the comfirmation of the reactant and product were changed as seen below:


The resulting transition structure for this calculation is shown below:
It can be seen that this has a structure close to the Boat comfirmation of cyclohexane and is the Boat TS for the Cope rearragement. Below is given some of the calculated energy data:
| Energy (Hartrees) | -231.6028 |
| Gradent on the Energy Surface (Hartrees) | 0.000011 |
| Sum of Electronic and Zero Point Energies (0K) | -231.4509 |
| Sum of Electronic and Thermal Energies (298K) | -231.4452 |
To insure both the Chair and the Boat transition states for the Cope rearragement had been found sucessfully, the vibrations of the molecule were looked at. It is known that a transition state should have one vibration at a negative frequency and that the motion of this vibration should be the motion fo the bonds forming/breaking in the reaction. The motion of the negative frequency in both the Chair TS and the Boat TS are shown below, ans show that these are the correct Chair TS and Boat TS:
| Transition State | Frequency | Vibration |
|---|---|---|
| Chair | 817.98cm-1 | 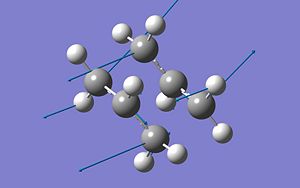
|
| Boat | 840.00cm-1 | 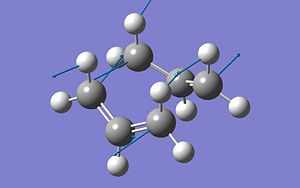
|
The two transition states have been found, but it is unknown which of the confirmations of 1,5 Hexadiene is required for a Cope rearragement to occur to reach one of these two transition states. As metioned above, it is predicted that the comfirmation is the Anti 2 comfirmation, but this needs to be proved. This can be found by doing a Intrinisic Reaction Coordinate (IRC) calculation. This done for both of the transition states, calculating the force constant once in the transition state. The IRC was also only calculated in one direction, due to the fcat that the reaction was symetrical (Product are the same as Reactants). It would be calculated in both directions if a rearragement resulted in the reactants being different from the products. The result of these calculations are shown below, as well as the comfirmation of 1,5 Hexadiene found by these calculations:




File:Chair101.molFile:Boat101.mol
Note. Chair TS Reactant is the comfirmation of 1,5 Hexadiene shown to be required by for a Cope rearragement to go though a Chair tansition state, where as the Boat TS Reactantis the comfirmation of 1,5 Hexadiene shown to be required by for a Cope rearragement to go though a Boat tansition state. Also, it can be seen that there is an anomally point on the IRC as calculated for the Chair TS due to the calculation failing.
It can be seen from the graphes above and the comfirmations of 1,5 Hexadiene that the optimisation fo the reactant for the Cope rearragement have not complete to give a minimum energy comfirmations of the reactant. This is suggested by the fact that on the both the IRC graphes (Boat TS and Chair TS IRCs) they do not reach a minimum (energy) point and the grandent on both IRCs does not reach zero (and the gradant at a minimum (energy) point is always zero). Therefore, two methods were tried inorder to try to find the minimum energy confirmations for the reactant of the Cope rearragement to result in a Chair TS and Boat TS. The first was to take the last comfirmation of 1,5 Hexadiene given on both the IRCs and to optimise them to their lowest energy comfirmation using the Hartree-Fock calculation method and the 3-21G basic set. The second method was to run the IRC calculation again, but this time to calculate the force constants at every step in order to find the confirmation of 1,5 Hexadiene require to under go a Cope rearragement via a Chair TS or a Boat TS. Both these method gave very similar structure for the confirmation of 1,5 Hexadiene required for a Cope Rearragement to occur via a Chair TS and a Boat TS and these comfirmations are shown below, along with the IRC graphs when calculating the force constant a every step:




File:Chair102.molFile:Boat102.mol
Note. Chair TS Reactant 2 is the comfirmation of 1,5 Hexadiene shown to be required by for a Cope rearragement to go though a Chair tansition state as found by the method decribed above, where as the Boat TS Reactantis the comfirmation of 1,5 Hexadiene shown to be required by for a Cope rearragement to go though a Boat tansition state as found by the methods above.
It can be seen that the confirmation of 1,5 Hexadiene required to under go a Cope rearragement via a Chair TS is the Anti 2 comfirmation. It can also be seen that the confirmation of 1,5 Hexadiene required to under go a Cope rearragement via a Boat TS is very similar in comfirmation to the Anti 2 comfirmation of 1,5 Hexadiene, showing the prediction that the confirmation of 1,5 Hexadiene required to under go a Cope rearragement is the Anti 2 comfirmation is valid.
Knowing the comfirmation and energy values of the reacatent (Anti 2) and the comfirmation and energies of the two possible transition states (Chair TS and Boat TS), it possible to predict which of the two transition states the Cope rearragment will precede by and the energy changes going from the react to the two possible transition states. This is shown in the table below, along with the energies for the Chair TS and Boat TS when calculated using the B3LYP calculation method and 6-31G d basic set in order to compare the calculation method/basic set combination results:
| Electronic Energy (HF 3-21G) | Sum of Electronic and Zero-point Energies at 0K (HF 3-21G) | Sum Electronic and Thermal Energies at 298K (HF 3-21G) | Electronic Energy (B3LYP 6-31G d) | Sum of Electronic and Zero-point Energies at 0K (B3LYP 6-31G d) | Sum Electronic and Thermal Energies at 298K (B3LYP 6-31G d) | |
|---|---|---|---|---|---|---|
| Chair TS | -231.6193 | -231.4667 | -231.4613 | -234.5589 | -234.4170 | -234.4090 |
| Boat TS | -231.6028 | -231.4509 | -231.4452 | -234.5450 | -234.4045 | -234.3960 |
| Anti 2 (Reactant) | -231.6925 | -231.5395 | -231.5325 | -234.6117 | -234.4692 | -234.4619 |
| HF 3-21G at 0K | HF 3-21G at 298K | B3LYP 6-31G d at 0K | B3LYP 6-31G d at 298K | Experimental at 0K | |
|---|---|---|---|---|---|
| Chair TS and Reactant | 45.68 | 44.68 | 31.06 | 33.17 | 33.5 |
| Boat TS and Reactant | 55.60 | 54.78 | 41.85 | 41.32 | 44.7 |
Note: 1 Hartree = 627.509 kcal/mol. HF reference to the the Hartree-Fock calculation method. Energy difference found between Sum ok Electronic and Zero-point Energies for 0K and the Sum Electronic and Thermal Energies for 298K
It can be seen that the energy difference is less between the reactant and the Chair TS compared to the reactant and the Boat TS, suggesting that the Cope rearragement goes though a Chair Transition state. It can also be seen that the calculations and the resulting energy difference for the more complex B3LYP 6-31G d calculation/basic set combination are closer to what is seen experimentally compared to the Hartree-Fock 3-21G calculation/basic set combination.
The Diels Alder Cycloaddition
Cis-butadiene reaction with ethene
Consider the Diels Alder Cycloaddition reaction below:

It can be seen that for this reaction to occur, there must be good orbital overlap between the pi-orbitals on both the cis-butadiene and the ethene molecule and that the phase of the orbital lobes interacting must be the same (positive to positive, negative to negative). For this to happen, the symmetry of the molecular orbitals interacting (HOMO and LUMO) on the two molecules must be the same with respect to the plane run down the molecule. To find the shape the molecular orbitals of moleculesand hence find their symmetry, computer calculations can be done when the structure of the molecule is being optimimised (as shown above) and will give the shape of the molecular orbitals. Below is given the HOMO and LUMO for Cis-butadiene and Ethene:




From the molecular orbitals above, it can be seen that both LUMO of Cis-butediene and HOMO of Ethene are symmetry with respect to a plane perpendicular to the carbon-carbon single in the former and the carbon-carbon double bond in the latter and it can also be seen that these orbitals could overlap with the correct phases and, as it is a HOMO and LUMO overlapping, the chemical reaction above could occur. The same is true if considering the HOMO of the Cis-butediene and the LUMO of the Ethene. Both of these orbitals are anti-symmetric with respect to the same plane decribed above and they can overlap with the same phase, allowing the reaction above to take place. However, one of these two pairs of molecular orbitals (LUMO of Cis-butediene/HOMO of Ethene and HOMO of Cis-butediene/LUMO of the Ethene) will form the HOMO of the transition state of this reaction and the other pair will form the LUMO of the transition state of this reaction and it can not be known which pair will result in which molecular orbital in the transition state from the information above. Knowing which molecular orbitals in the reactants results in which molecular orbital in the transition state is important as in reaction where more than one isomer can form, this imformation may help determine which product will predominate by considering the orbital overlap between reactants and in the transtion state.
In order to answer the question posed above (which reactant orbitals results in which orbitals in the transition state of the Diels Alder reaction given above), first the confirmation of the transition state has to be found. This was done by building a structure in the computer interface which was thought to be close to that of the transition state and then this structure was optimised to a TS Berny, using the AM1 Semi-Empirial calculation and calculating the force once. The resulting structure is shown below:
To comfirm that the correct structure had been calculated for the Diels Alder transition state, it was checked that there was one negative frequency and that the motion of this frequency was formation of the bonds shown to be make in the Diels Alder reaction scheme above. It was found that there was one negative frequency occuring at -956.23cm-1 and this is shown below:

This vibration above does shown the formation of the bonds seen to be forming the reaction scheme of the Diels Alder cycloaddition seen above and comfirms the fact that the correct structure of the transition state has been calculated. The vibration given above also provides other information. It can be seen from the vibrataion above that both the bonds forming are forming at the same time, the bonds form synchronous. The bond forming vibration can also be compared to the lowest positive frequency vibration to see if they are similar. The lowest positive frequency vibration occurs at 147.29cm-1 and is shown below:

It can be seen that this vibration is a bending type of vibration, unlike the bond forming vibration which a stretching vibration. It can seen when comparing the two vibrations, the bond forming vibration is symmetric (hence why the bonds form synchronous) and the displacement vectors for the bond forming vibration are large compared to the lowest positive frequency vibartion which is antisymmetric (so if this was the bond forming vibration, the bonds would form asynchronous) and the displacement vectors for the vibration are small.
Below is given a table of bond lengths seen in the transitions state of the Diels Alder reaction. The Diels Alder transition state was optimised again using the Hartree-Fock calculation method 3-21G basic set and the bond lengths found by this calculation are also shown below:
Uning the optimised structure of the transition state, it is possible to find the shape of the HOMO and LUMO of the transition state. These are shown below:


It can be observed from the molecular orbitals above for the transition state that the HOMO is antisymmetric (with regards to a plane perpendicular to the carbon-carbon double bond) and is formed from the HOMO of the cis-butadiene and the LUMO of the ethene and that the LUMO is symmetric and is formed from the LUMO of cis-butadiene and the HOMO of the ethene. As explainned above, these MO for the transition state can form, allowing the reaction to take place, due to the good overlap between the molecular orbitals of the reactants and the fact that when the orbitals overlap they are all of the same phase in both cases.
Cyclohexa-1,3-diene reaction with Maleic Anhydride
Considering the reaction shown below, it is known that one of two product could be formed, either a exo or endo product:
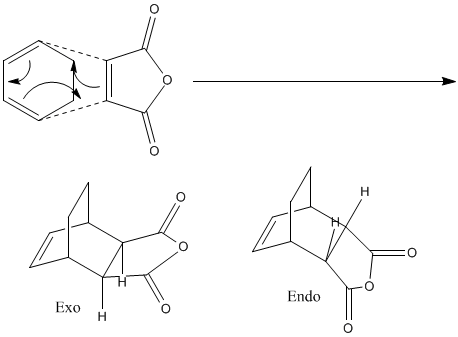
It is known that the exo product is lower in energy, but the major product of this reaction is the endo product. This suggests that this reaction is under kinteic control and that the energy of the transition to the endo product is lower than the transition state leading to the exp product.
In order to investigate whether the exo transition state (Exo TS) is higher in energy than the endo transition (Endo TS) state and to explain why this might be the case, an approximate structure of both the possible transition states were built into the computer interface and were optimised to TS Berny, calculating the force constants once and using the AM1 Semi-emprical calculation method. The resulting structures of the transition states are shown below:
File:DATS Exo.molFile:DATS Endo.mol
As normal, the transitions states were checked to be in the correct comfirmation by checking they had one negative frequency that had a motion relating to the bonds forming in the reaction. These two neative frequencies (one for each transition state) are shown below, along with the frequency which they occur at:


It can be seen that the negative frequancy found for each of the transition states does corespond to the bond forming proess seen in the reaction scheme above, comfirming that the correct structures for the two transition states had been found. The two transition states were furher optimised by optimising to TS Berny and calculating the force constants once, but using the Hartree-Fock calculation method and 3-21G basic set and using the B3LYP calculation method and the 6-31G d basic set. The energies found by these three methods are shown below:
| Energy Term | Exo TS | Endo TS |
|---|---|---|
| Sum of Electronic and Zero Point Energies | 0.1349 | 0.1195 |
| Sum of Electronic and Thermal Energies | 0.1449 | 0.1298 |
| Total Energy | -0.0504 | -0.1298 |
| Energy Term | Exo TS | Endo TS |
|---|---|---|
| Sum of Electronic and Zero Point Energies | -605.4240 | -605.4331 |
| Sum of Electronic and Thermal Energies | -605.4145 | -605.4236 |
| Total Energy | -605.6190 | -605.6281 |
| Energy Term | Exo TS | Endo TS |
|---|---|---|
| Sum of Electronic and Zero Point Energies | -612.5105 | |
| Sum of Electronic and Thermal Energies | -612.5001 | |
| Total Energy | -612.6915 |
The data in the tables above comfirm the fact that the transition state leading to the Exo product is higher in energy than the transition state leading the Endo product, explaining why the Endo product is seen as the major product of the reaction, as this reaction is under kinteic control. However, these results do not explain why the Endo TS is lower in energy than the Exo TS.
One charateristic which many explain the energy difference between the Exo TS and the Endo TS is the bond lengths and the distance between the O=C-O-C=O unit and the -CH2-CH2- unit in the Exo TS and the distance between the O=C-O-C=O unit and the -CH=CH- unit in the Endo TS (These 2 lengths will be called length x). The last distance decribed, length x, is important as it is the distance between the two large groups and so the shorter the distance between them, the greater the steric slash, causing the energy of transition state to increase. The distance for these lengths for both the Exo TS and Endo TS are shown below for the different calculation methods and basic sets:
| Structural Parameter | Exo TS | Endo TS |
|---|---|---|
| Length x | 3.34Â | 3.31Â |
| C-C Forming bond length | 2.17Â | 2.17Â |
| C-OC Bond length | 1.41Â | 1.41Â |
| C=C Bond length (Double bond braking) | 1.39Â | 1.39Â |
| C=C Bond length (Double bond forming) | 1.40Â | 1.40Â |
| Structural Parameter | Exo TS | Endo TS |
|---|---|---|
| Length x | 3.32Â | 3.10Â |
| C-C Forming bond length | 2.27Â | 2.30Â |
| C-OC Bond length | 1.40Â | 1.40Â |
| C=C Bond length (Double bond braking) | 1.37Â | 1.39Â |
| C=C Bond length (Double bond forming) | 1.40Â | 1.40Â |
| Structural Parameter | Exo TS | Endo TS |
|---|---|---|
| Length x | 3.34Â | 3.31Â |
| C-C Forming bond length | 2.17Â | 2.17Â |
| C-OC Bond length | 1.41Â | 1.41Â |
| C=C Bond length (Double bond braking) | 1.39Â | 1.39Â |
| C=C Bond length (Double bond forming) | 1.40Â | 1.40Â |