Rep:Mod:ThelineCLRW
Computational Lab
Using Gaussview 5.0, the optimisation of a number of molecules: NH3, N2, H2 were run in order to obtain a reaction energy of the Haber-Bosch process; along with an investigation into the molecular orbitals and reactivity of CN-
Ammonia
Ammonia Optimisation Data
Calculation method:RB3LYP
Basis-Set:6-31G(d,p)
Charge: 0
Spin: Singlet
Total energy: -56.55776873 a.u
RMS Gradient: 0.00000485
Dipole moment: 1.8467 a.u
Point Group: C3v
Bond Distance: 1.01798 Å
H-N-H Bond Angle: 105.741°
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Optimised NH3 molecule
Ammonia |
Vibrational Analysis
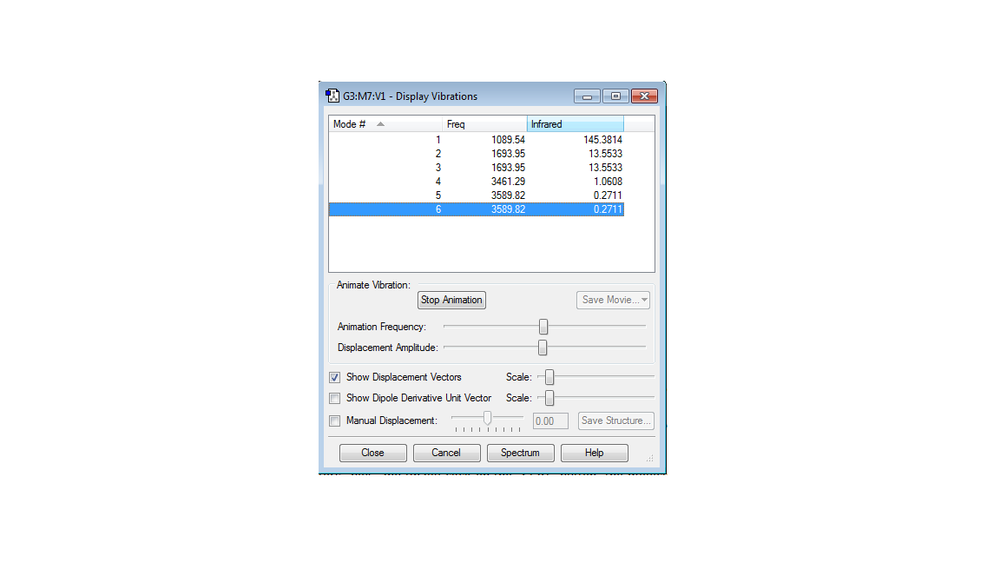
Questions
Expect to see 6 modes of Vibration for NH3 from 3N-6 rule
Modes 2 and 3, 5 and 6 (see numbers in table above) have the same energy
Modes 1 and 2 and 3 are bending vibrations, Modes 4,5 and 6 are stretching vibrations
Mode 4 is a highly symmetric Stretching mode
Mode number 1 is the 'Umbrella mode' the molecule appears to 'unfold' outwards and inwards like an umbrella
2 bands are expected to be seen as 4 of the modes have such a low frequency they have bands that will be 'lost' in a spectrum
Nitrogen has a charge of -1.125 and each hydrogen has a charge of +0.375, this is expected as the ammonia molecule is neutral and the positive charges of the hydrogens balance with the negative charge on the nitrogen atom
This is also expected as nitrogen has a greater electronegativity than hydrogen, so it is expected that nitrogen will be pulling electron density away from the hydrogens
Nitrogen
Nitrogen optimisation Data
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Charge: 0
Charge: Singlet
Total energy: -109.52412868 a.u
RMS Gradient: 0.00000365
dipole moment: 0.0000
Point group: D∞h
N-N bond Angle: 180°
N-N bond length:1.10550 Å
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
Vibrational anaylsis
Optimised N2 Molecule
Nitrogen |
Hydrogen optimisation data
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Charge: 0
Spin: Singlet
Total energy: -1.17853936 a.u
RMS Gradient Norm: 0.00000017
Dipole Moment: 0.0000
Point Group: D∞h
H-H bond angle: 180°
H-H bond distance: 0.74279 Å
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Vibrational analysis

Optimised H2 Molecule
Hydrogen |
Haber-Bosch Process
-Below are summarised data used from analysis to calculate the change in energy when hydrogen and nitrogen react to make ammonia
E(NH3)= -56.55776873 a.u
2*E(NH3)= -113.11553746 a.u
E(N2)= -109.52412868 a.u
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= (-113.11553746)-(-109.52412868+(-3.53561808)) = -0.0557907 a.u
ΔE= -0.0557907 a.u = (-0.0557907*2625.5)Kj/Mol = -146.47848285 Kj/Mol
ΔE= -146.48 Kj/Mol (2 d.p)
Therefore, as the reaction is exothermic, the ammonia product is lower in energy than the starting reagent gases. This means ammonia is more stable than the reacting gases
Cyanide Anion
Optimisation Data
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Charge: -1
Spin: Singlet
Total energy: -92.82453151a.u
RMS Gradient norm: 0.00012733
Dipole moment:0.5235
Point Group: C∞
C-N bond length: 1.18418 Å
C-N bond angle: 180°
Item Value Threshold Converged? Maximum Force 0.000221 0.000450 YES RMS Force 0.000221 0.000300 YES Maximum Displacement 0.000099 0.001800 YES RMS Displacement 0.000139 0.001200 YES
Vibrational analysis

Molecular Orbitals
The Cyanide anion is made up of a carbon and a nitrogen
The electronic configuration of carbon is 1s2 2s2 2p2
The electronic configuration of nitrogen is 1s2 2s2 2p3
Therefore, there are 9 Atomic orbitals which are occupied which combine to form 9 Molecular orbitals
MO diagram for CN- as reference[1]

Molecular orbital pictures
1σ - This is the lowest molecular orbital formed from Carbon and nitrogen bonding, as seen below, this molecular orbital has no observational overlap, the energy is also considerably lower -14.00395 a.u than any of the other molecular orbitals, as a result we can consider the 1σ to be a non-bonding orbital that is too deep to contribute to reactivity

2σ* - This is lowest molecular anti-bonding orbital, this molecular orbital also has no observational overlap, the energy is also considerably lower -9.86723 a.u than any of the other molecular orbitals just like the 1σ orbital and as a result we can consider the 2σ* to be a non-bonding orbital

3σ - This is the first bonding molecular orbital formed from the carbon and nitrogen 2s orbitals as seen below. It has an energy of -0.56952 a.u. This molecular orbital has clear complete overlap with a larger lobe of electron density on the nitrogen atom, nitrogen is more electronegative than carbon so is expected to contribute more to the bonding interaction as seen in the image. This is a bonding molecular orbital however it doesn't appear to be in the HOMO (highest occupied molecular orbital) or LUMO (lowest unoccupied molecular orbital) region. Therefore, it is not expected to take part considerably in any reactivity of CN-

4σ* - This is the first anti-bonding molecular orbital formed the 2s orbitals of carbon and nitrogen. It has an energy of -0.10628 a.u. This molecular orbital has very good overlap, with a much larger green lobe of electron density observed on the carbon atom. Carbon is less electronegative than nitrogen so it is expected that carbon contributes more to the anti-bonding interaction of this 4σ* molecular orbital. However, just like the 3σ it appears still too low in energy to contribute significantly to the reactivity of CN-

1π - The 1π orbitals are degenerate molecular orbitals formed from the sideways overlap of two of the 2p orbitals from both carbon and nitrogen. The degenerate molecular orbitals both have energies of -0.01694 a.u . Interestingly however, is that the 1π orbitals usually are of higher energy than the σ overlap from 2p orbitals, this will be explained when analysing the 5σ molecular orbital. However, once again the energies of these MO's are considerably lower than the HOMO and LUMO so it is not expected the π molecular orbitals take part significantly in reactivity.


5σ - This is the HOMO of the cyanide anion. This molecular orbital is formed from the end on overlap of the remaining 2p orbitals of carbon and nitrogen and has a large electron density on and between the atoms. The interesting shape of the molecular orbital that appears reminiscent of an sp hybrid molecular orbital which is formed from mixing of the 3σ and the 5σ molecular orbitals. This mixing destabilises the 5σ orbital and stabilises the 3σ orbital. This moves the 5σ up in energy which pushes it higher than energy compared to the 1π orbitals. As this molecular orbital is the HOMO and is a sigma bond, it is expected that this molecular orbital will take part in nucleophilic attack of electrophilic species in reactions and indeed this reactivity hypothesis is confirmed in nucleophilic addition of cyanide anions to carbonyls to form cyanohydrins. This diagram also shows why the cyanide bonds by the carbon atom and not the nitrogen atom as the carbon atom contains the most electron density as shown in the image of this molecular orbital and hence will be the part of the molecule most likely to interact with the LUMO of the C-O π* of the carbonyl atom [2]

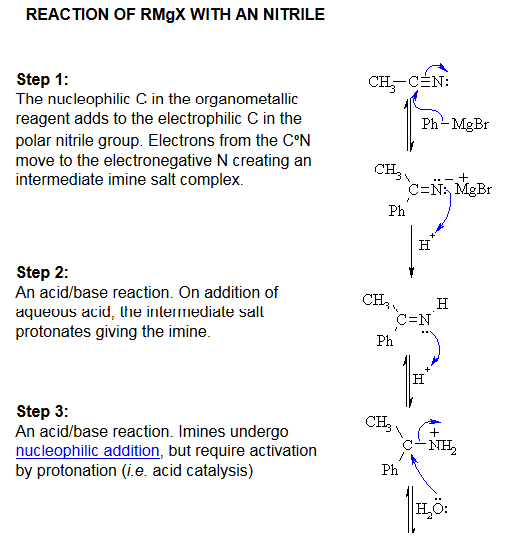
2π* - These are the LUMO's of the cyanide anion as they are degenerate π anti bonding orbitals. These molecular orbitals are expected to be involved in chemical reactions where a very High energy HOMO of an even more nucleophilic species can donate electrons into this LUMO of the cyanide anion. One such example of this is in the reaction of a grignards reagent(phenyl magnesium bromide) which attacks one of the 2π* molecular orbitals to form an imine salt. This is the first step in the reduction of a nitrile to a ketone [3]


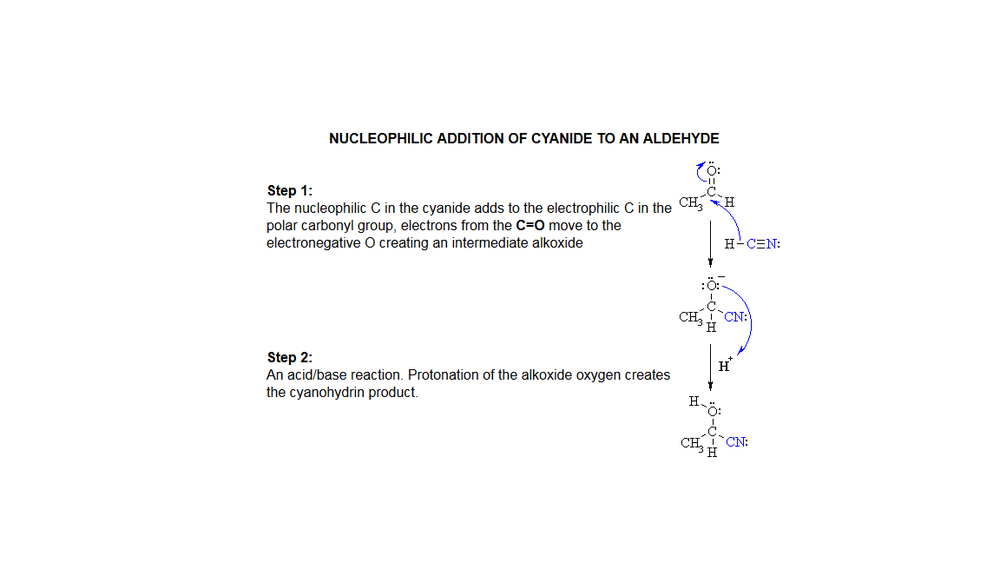
Reaction of a cyanide anion with methanal[2]
REACTION OF RMgX WITH A NITRILE[3]
References
- ↑ http://chemistry.stackexchange.com/questions/18449/cyanide-ion-non-bonding-lone-pair
- ↑ 2.0 2.1 http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch17/ch17-3-2-1.html
- ↑ 3.0 3.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedReductionref
