Rep:Mod:TSYts15
Introduction
To begin with, Optimisation and Frequency calculation are particularly fundamental at Computational Chemistry. These calculation provide important insight to kinetic and thermodynamic parameters in a chemical reaction.
A Potential Energy Surface (PES) is illustrated below.
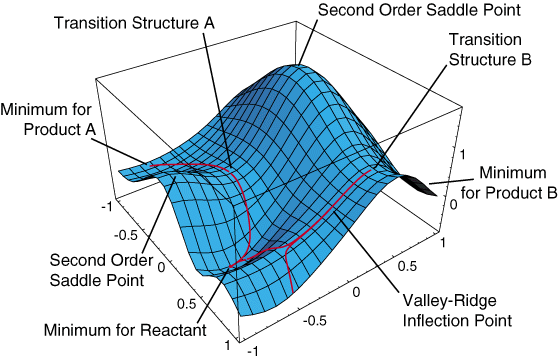
The minima are represented by A and B which corresponds to reactants and products. Hence ,the reaction path is defined between the two minima. From the PES, reactants pass through the activation barrier, the peak of energy profile (first order saddle point), along the reaction path. Hence, a transition state is the first order saddle point on a PES. The vibration calculation of a transition state demonstrates one negative frequency which indicates a negative force constant. This means that energy is at maximum in only one direction of nuclear-configuration space. While the energy is remain minimum in all other orthogonal directions. Therefore, it is necessary to verify the correct TS by performing frequency calculation plus geometry optimisation at the same computation basis set in this exercise. The imaginary frequency corresponds to the translational motion of transformation from reactants to products.
These can be quantified by mathematical concept as shown in Intro: Table 1 Gradient and curvature at mimimum and transition state.[1]
Nf710 (talk) 21:54, 29 November 2017 (UTC) Firstly in Guassian we workin in the space of the 3N-6 degrees of freedom and these are the coorodinates to which we find the forst constants. (its is actually he normal modes which is 3n-6 of these degrees of freedom)
| Minimum | Transition state | |
|---|---|---|
| Gradient | ||
| curvature | ||
In addition, frequencies allow calculation on the second derivatives of the optimised system three-dimentional matrix. The calculated second derivatives give the force constants (formula 1). As a result, frequency can be calculated from the force constant (formula 2)
Nf710 (talk) 21:54, 29 November 2017 (UTC) I dont know what you mean by the optimised 3 dimensional matrix
Formula 1. Force Constant
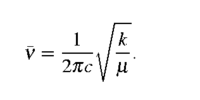
v is frequency , c, the speed of light, the force constant, and μ the reduced mass
Formula 2. Frequency Calculation
Moreover, both basis set of PM6 and B3LYP were used to provide optimisation result. Hatree-Fock, B3LYP method has a great advantage on treating electron correlation quite well whereas this is not the case for basis set PM6. Electron correlation is particularly relevant to transition state calculations and hence, providing a more accurate calculation. As a result, in exercise 2, B3LYP method is used in addition to PM6 method. Using PM6 basis set alone may yield incorrect prediction of the reaction. In contrast, semiempirical method, PM6 is an approximate verision of Hatree-Fock which reduce computation effort significantly at the expenses of accuracy. Only valence electrons are considered and a minimum basis set is used for the calculation. Semiempirical method, PM6, eliminates some two-electron or one-electron integral and insert empirical parameters to make up the neglected integral. As a result, Semiempirical PM 6 is well peformed in Exercise 1 and 3 since previous experimental data is available to correlate with. Another advantage of PM6 Basis set is Quantum Mechanics based calculation which is more robust than force fields.
Nf710 (talk) 21:54, 29 November 2017 (UTC) OK PM6 is correct, well done. good understanding there. B3LYP is a DFT method which used also a HF method to calculate the electron correlation. So techically it is a hybrid method.
Exercise 1: Reaction of Butadiene with Ethylene
Optimization Result
| Butadiene | Ethylene | Transition States | Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
The Table 1 shows the result of optimisation of reactants, TS and product at PM6 level respectively. Correct TS was further confirmed by frequency calculation and IRC.
Frequency
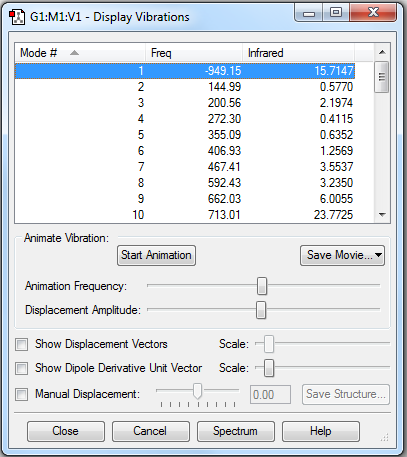
From Fig. 2, a negative frequency is shown in the final optimised TS. This indicates imaginary vibrational frequency associated with a TS which corresponds to the translational motion of carbon. At Gaussian, second derivative of the energy with respect to the nuclear position is calculated by the frequency. Hence, the first negative frequency indicates one negative second derivation, which confirms a transition state (the highest point on the minimum energy path linking the reactants and products)[2]. High frequency, greater than |100-1|, is consistent with this kind of molecule. As a result, this represents a correct TS is obtained from final optimisation . Additionally, this plays an important part in defining chemical reactions. One negative frequency also indicates the presence of single TS which illustrates a well-defined reaction profile and complete reaction.
IRC
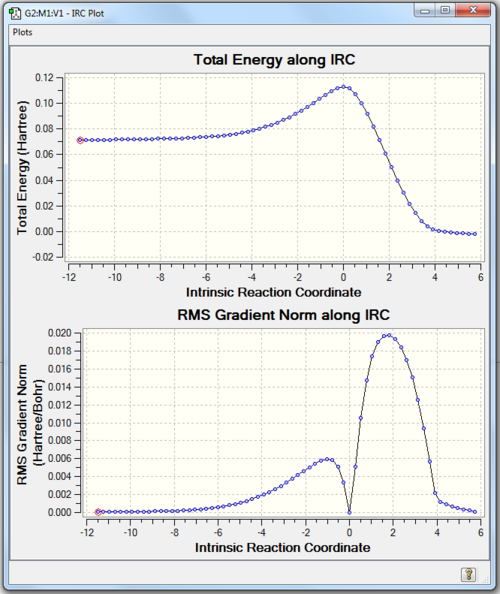
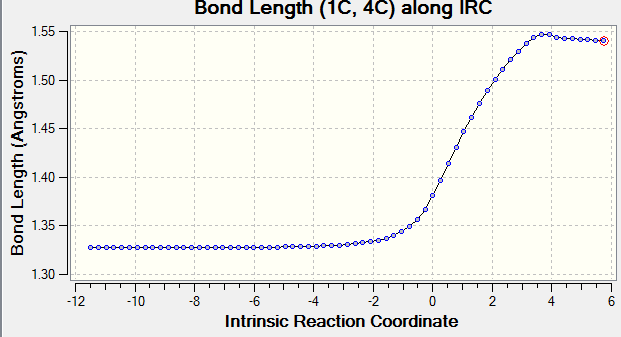
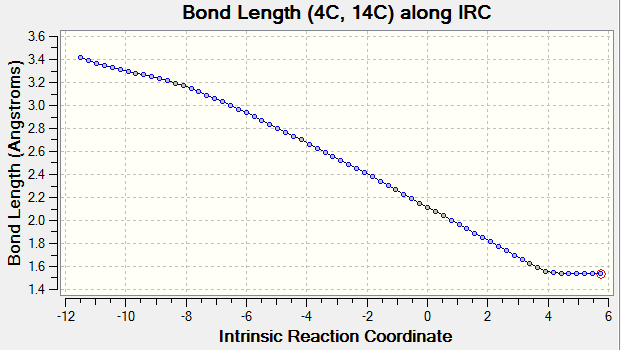
Transition state geometries can be connected to ground state geometries with an Intrinsic Reaction Coordination calculation (IRC) performed by Gaussian. In Fig 3., the downhill path from the transition is followed in forward direction. A well defined and asymmetric Free Energy Surface as shown in Fig. 3 confirms that TS is correctly optimised.
MO Analysis
MO diagram for the formation of butadiene/ethene TS is illustrated in Fig. 4 MO Diagram for the formation of Butadiene/ Ethene. It is correlated with the actual MOs are performed by Gaussian at Basis Set PM6. It is shown that TS LUMO +1 has energy lower than expected (stabilisation) and TS HOMO has energy higher than expected (destabilisation). Hence, this suggested that there is significant mixing between orbitals. In addition, it is important to note that the splitting between HOMO and LUMO of Butadiene (Diene) is much smaller than that of Ethene (Dienophile). This can be explained by the extra stabilisation of the conjugated system in Butadiene. From the MO Diagram, it is predicted that reaction maybe less efficient due to the large energy difference between the LUMO of diene and HOMO of dienophile. It is suggest that addition of electron donating group on the Diene will lower the energy of LUMO of diene and hence interact more strongly with HOMO of dienophile.
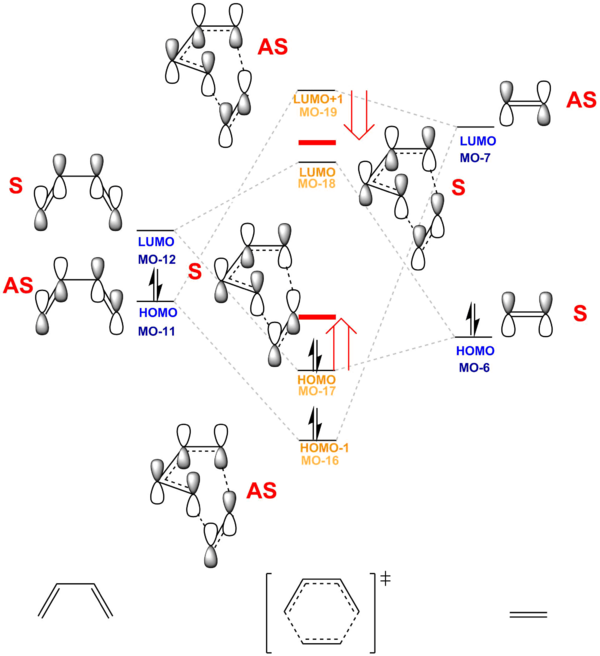
(Fv611 (talk) Your MO combinations are correct, but the energy levels are not. You have correctly calculated the energies of each MO, why not adjust your MO diagram accordingly? For example, you have calculated the energy of the Ethene HOMO to be -0.39, and that of the TS HOMO-1 to be -0.32, yet you draw the TS HOMO-1 lower in energy than the Ethene HOMO.)
| Orbital type | Butadiene | Ethylene | ||||||
|---|---|---|---|---|---|---|---|---|
| HOMO |
|
| ||||||
| LUMO |
|
|
| HOMO-1 | HOMO | LUMO | LUMO+1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
From the MO diagram, it can be concluded that only reactants' MOs of the same symmetry are allowed to react together. Therefore, symmetry of MOs are directly related to the extent of Orbital overlap since reaction cannot proceed with zero orbital overlap. Reaction can only proceed when there is non-zero orbital overlap. Therefore, only interaction between SAME symmetry HOMO and LUMOs (non-zero orbital overlap) are allowed to react. The result is illustrated by the table below.
| Symmetric-Antisymmetric | Symmetric-Symmetric | Antisymmetric-Antisymmetric | |
|---|---|---|---|
| Overlap Intergral | Zero | Non-zero | Non-zero |
| Reaction | Forbidden | Allowed | Allowed |
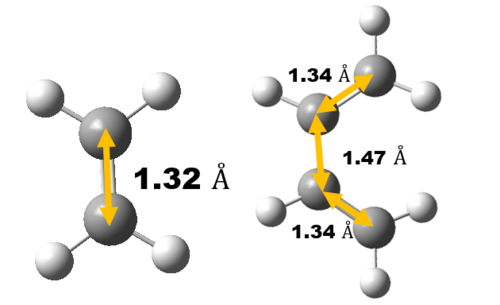
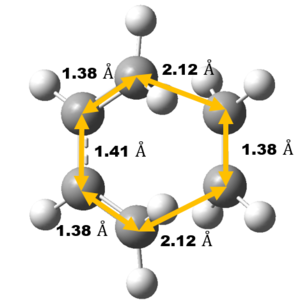
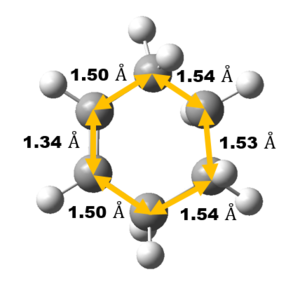
Bond Length Analysis
| Reactant | Transition States | Product | Literature value |
|---|---|---|---|
|
|
|
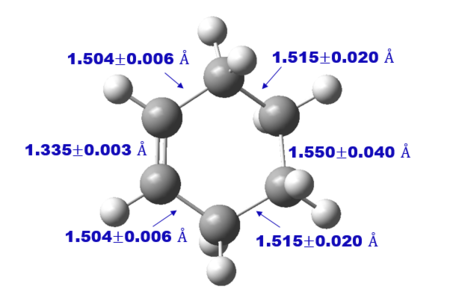
Experimental carbon carbon bond length of cyclohexene is consistent with the literature value as shown in Table 3 which confirm correct structure of TS and Cyclohexene Product is obtained. Comparing the Van der Waals radius of C atom and the length of the partly formed C-C bonds in the TS, a much lower C-C bond length at TS indicates formation of bond. Typically, sp2 C=C bond lengths is much shorter than sp3 C-C bond length as shown in table 3 due to a higher bond order and hence, closer the Carbon atoms are.
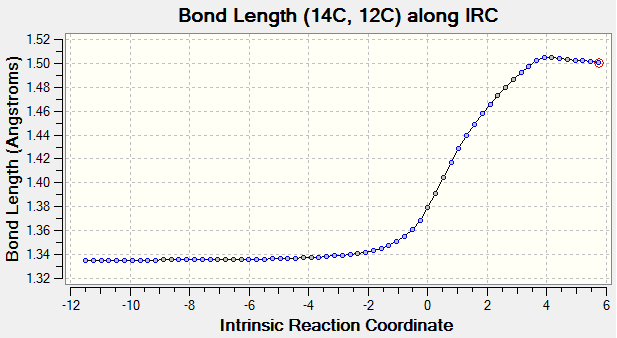
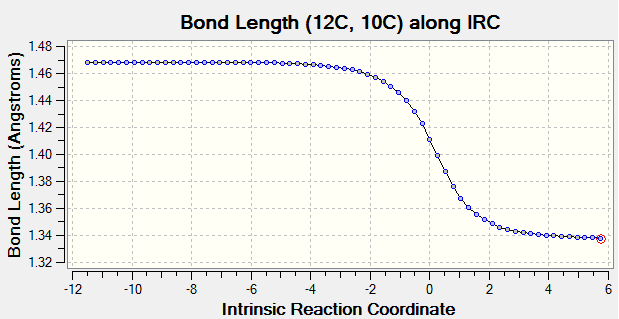
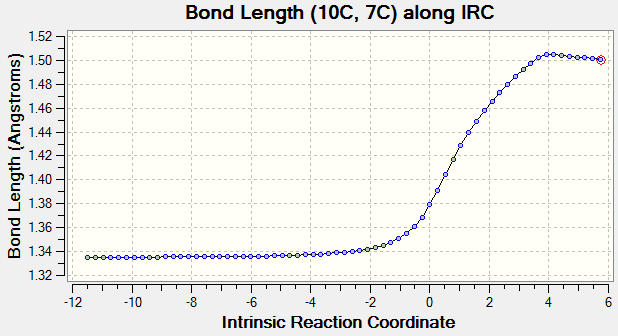
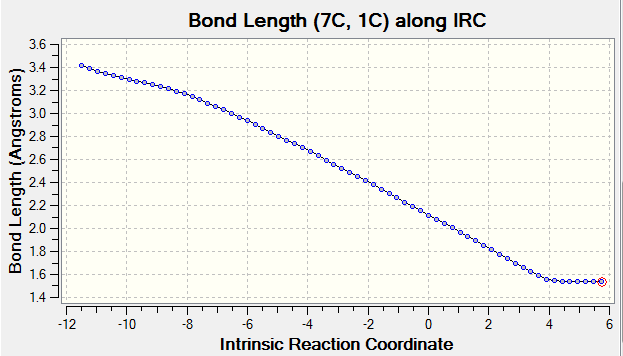
As reaction proceed, bond length corresponds to different carbon atoms change which the result is shown in Table 4.
(Fv611 (talk) There could have been a slightly more wordy discussion, this is more a report on calculated bond lengths than an analysis of what they mean.)
Vibrational Analysis
The vibration that corresponds to the reaction path at transition state is -949.15 cm-1. In the transition state of this reaction, the two forming bonds have the same lengths. Hence, this confirms the synchronous mechanism where two new bonds are formed simultaneously.
Vibrational Analysis of TS |
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
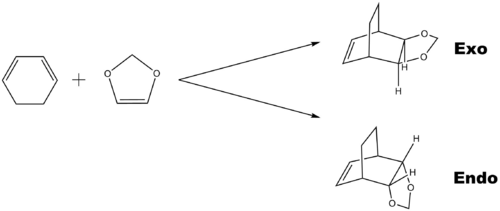
Optimisation Result
Table 5 shows the result of the Optimisation of reactants; endo and exo TSs. It was first optimised at PM6 method then a more accurate method, at B3LYP/6-31G(d) Basis Set
| Basic Set | Cyclohexadiene | 1,3-Dioxole | Exo TS | Endo TS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM6 | ||||||||||||
| B3LYP/6-31G(d) |
Frequency Analysis
A correct TS is further confirmed by the frequency analysis. Only one negative frequency at Exo and Endo TS indicates a saddle point reached which confirms the correct TS obtained.
MO Analysis
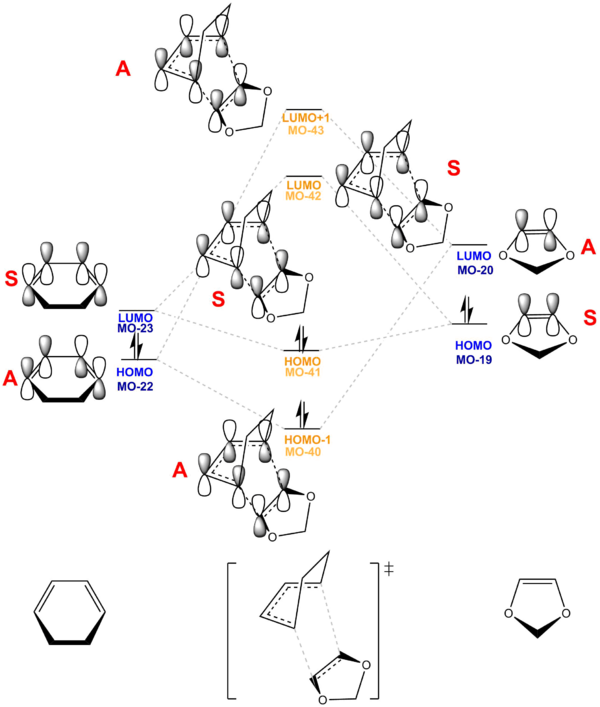
Figure 12 and 13 shows the MO digram for the Diels-Alder reaction (Exo and Endo) , which correlate with the actual MOs performed by Gaussian at B3LYP/6-31G(d) basis set.
 |
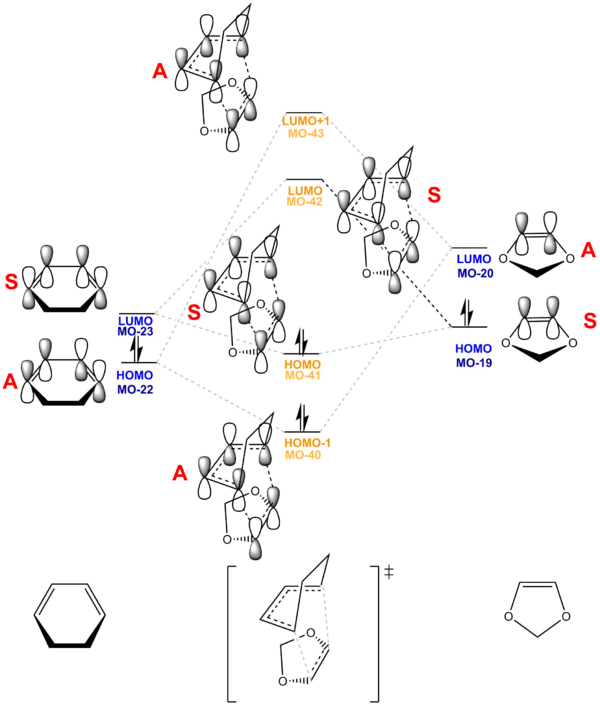 |
(Fv611 (talk) Like in the previous exercise, your pairings are correct but you haven't correlated the positions of your MOs with their computed energies. The dienophile LUMO is the orbital with the highest energy, and the diene HOMO is the orbital with the lowest energy. Additionally, it would have been nice to discuss the difference between Exo and Endo case.)
| Orbital type | Exo TS | Endo TS | ||||||
|---|---|---|---|---|---|---|---|---|
| HOMO-1 |
|
| ||||||
| HOMO |
|
| ||||||
| LUMO |
|
| ||||||
| LUMO+1 |
|
|
This reaction is an inverse demand DA reaction since diene is less electron rich than dienophile in this reaction. The electron-donating oxygen group donate electrons into the double bond dienophile, making it more electron rich.
Hence, the Frontier Molecular Orbitals the LUMO of Cyclohexadiene (Antisymmetric) interact with the HOMO of 1,3-Dioxole, dienophile, are closer in energy than that of HOMO of diene and LUMO of dienophile. As a result, they interact most strongly and form most energetically favourable bond formation. [6] As a result, this reaction is an Inverse Demand DA reaction.
The HOMO-1, HOMO, LUMO, LUMO+1 TS MOs shows A-S-S-A symmetry which further confirm it is an Inverse Demand DA reaction. (Fv611 (talk) This isn't true, as the reaction discussed in exercise 1 also has A-S-S-A symmetry but is a normal electron demand case.)
Reaction Thermodynamics
| Basic Set | Cyclohexadiene | 1,3-Dioxole | Exo TS | Endo TS | Exo Product | Endo Product | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | |
| PM6 | 0.116878 | 306.863212 | −0.0522760 | −137.25064846 | 0.138903 | 364.689854 | 0.137941 | 362.164123 | 0.037972 | 99.6954936 | 0.037807 | 99.2622861 |
| B3LYP/6-31G(d) | −233.324375 | −612593.19323 | –267.068645 | –701188.78086 | –500.329165 | –1313614.3228 | –500.332147 | –1313622.152 | –500.417322 | –1313845.779 | −500.418691 | –1313849.3733 |
| Activation Barrier/kJ/mol | Reaction Energy/kJ/mol | |
|---|---|---|
| Exo Reaction pathway | +168 | -63.80491 |
| Endo Reaction pathway | +163 | -67.39921 |
Table 8. shows the result of the Activation Barrier and reaction energy on the reaction of Cyclohexadiene and 1,3-Dioxole. Activation Barrier is calculated by the difference between the energy of product and TS. While Reaction Energy is the differences between product and reactants energies. It is important to note that Basis set of B3LYP/6-31G(d) is used to provide more accurate calculation.
Kinetic favourable of the products can be determined by the Activation Barrier. As shown in Table 8, Endo reaction pathway has a lower Activation energy and hence Endo product is more kinetically favourable. This can be further explained by secondary (non-bonding) orbital interaction in the endo TS HOMO as shown in Table 9. Secondary orbital interaction between diene and the dienophile substituent's π system lowers the energy of the endo TS and hence, lower activation barrier and the reaction proceed much faster towards endo-product. [7]As a result, endo product is more kinetically favourable. On the other hand, there is no such interaction between Diene and the substituents of dienophile in exo TS as shown in the HOMO of exo TS in table 9. Hence, there is no stabilisation of the exo Transistion state. Only primary (bonding) interaction present in the Exo-TS.
Moreover, endo product is more thermodynamically favourable. Comparing the reaction energy between Endo- and Exo- reaction pathway, more negative number is obtained at the reaction towards the formation of Endo-product. Hence, this confirms that the endo-product is more thermodynamically stable. In fact, less sterically-hindered Exo-TS is expected to be more thermodynamically favourable. This unexpected result of Endo-Product being more more thermodynamic favourable of can be explained by Hammonds Postulate. Since this is an endothermic reaction (bond forming), according to Hammonds Postulate, it is expected that TS is more product like. As a consequence, stabilisation by secondary (non-bonding) orbital interaction will also apply to the endo-product.
| HOMO of Exo TS | HOMO of Endo TS | ||||||
|---|---|---|---|---|---|---|---|
|
|
As a result, it can be concluded that inverse electron demand Diels Alder reaction favours endo-Product in terms of thermodynamics and kinetic argument.
Nf710 (talk) 22:02, 29 November 2017 (UTC) Well done your energies look correct and you have come to the correct conclusion. Good understanding of the electon demand of the reaction
Exercise 3: Diels-Alder vs Cheletropic
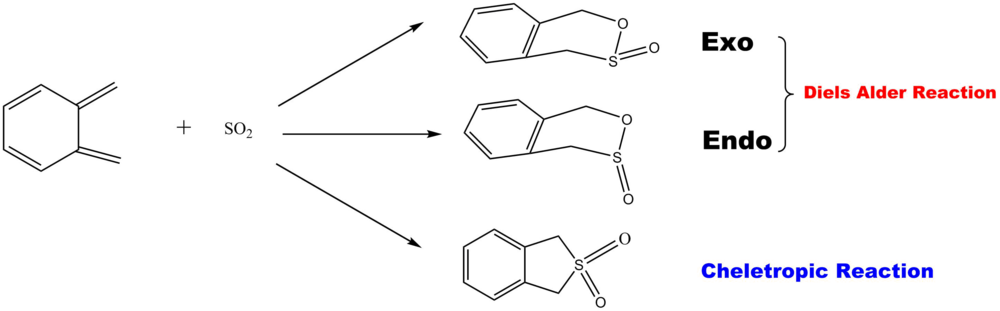
Optimisation Result
| Xylylene | SO2 | ||||
|---|---|---|---|---|---|
Table 10. shows the optimisation result of reactants. It is confirmed that correct reactants are obtained as there is no imaginary frequency.
| Exo TS | Endo TS | Cheletropic TS | ||||||
|---|---|---|---|---|---|---|---|---|
Table 11. shows the optimisation result of the exo TS, endo TS and Cheletropic TS at PM6 Basis Set.
IRC Analysis
The reaction coordinate for each path are illustrated in the following animation according to an IRC calculation.
Reaction Thermodynamics
| Basic Set | Xylylene | SO2 | Exo TS | Endo TS | Cheletropic TS | Exo Product | Endo Product | Cheletropic Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | |
| PM6 | 0.178070 | 467.522821 | −0.118614 | −311.421081 | 0.092078 | 241.750807 | 0.090560 | 237.765298 | 0.099062 | 260.087301 | 0.021455 | 56.3301068 | 0.021706 | 56.9891073 | −0.000002 | −0.0052510004 |
Table 12. shows the result of the reaction barrier and reaction energy on the reaction of Cyclohexadiene and 1,3-Dioxole. Activation Barrier is calculated by the difference between the energy of product and TS. While Reaction Energy is the differences between product and reactants energies. It is important to note that Basis set of B3LYP/6-31G(d) is used to provide more accurate calculation.
(B3LYP is a DFT functional, 6-31g(d) is the basis set. You are also not using B3LYP here, so be careful when you copy text from previous sections. Tam10 (talk) 11:24, 24 November 2017 (UTC))
| Activation Barrier/kJ/mol | Reaction Energy/kJ/mol | |
|---|---|---|
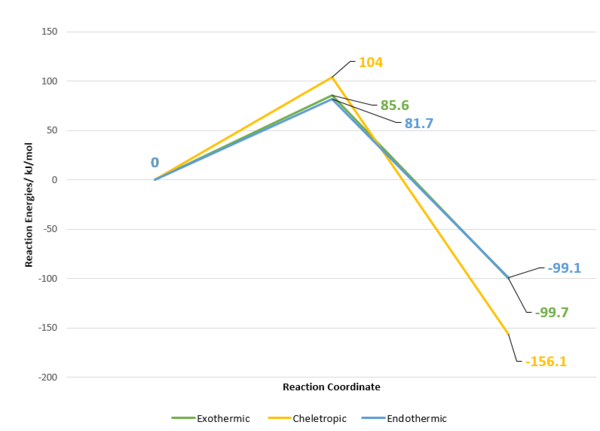
| Exo Reaction pathway | +85.6 | -99.7 |
| Endo Reaction pathway | +81.7 | -99.1 |
| Cheletropic Pathway | +104.0 | -156.1 |
Table 13 shows that the activation and reaction energies for each reaction pathway. It can be concluded that cheletropic pathway is most thermodynamically favorable as the Gibbs free energy of the whole reaction is most negative. However, reaction pathways towards the formation of Cheletropic Product has the highest activation energy. In contrast, Endothermic product is most kinetic favorable. Since it has the lowest activation barrier among other pathway, the rate of reaction towards the formation of endothermic product is the highest. This is demonstrated by figure X the reaction profile for the three pathways.
(You haven't mentioned the driving force behind these reactions Tam10 (talk) 11:24, 24 November 2017 (UTC))
Extra
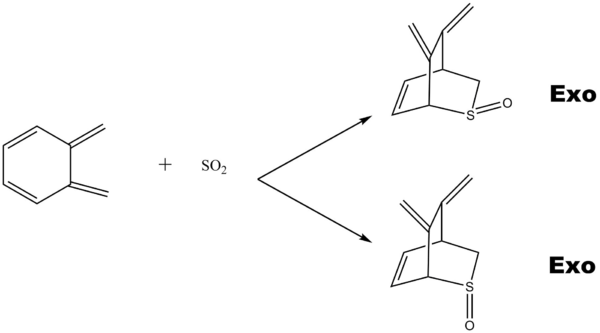
The Second cis-butadiene in o-xylylene can undergo another Diels-Alder reaction which illustrated in figure 16.
| Basic Set | Xylylene | SO2 | Exo TS | Endo TS | Exo Product | Endo Product | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | Energy/ Hatress | Energy/ kJ/mol | |
| PM6 | 0.178070 | 467.522821 | −0.118614 | −311.421081 | 0.105053 | 275.816673 | 0.102071 | 267.9874309 | 0.067304 | 176.7066655 | 0.065610 | 172.2590681 |
| Activation Barrier/kJ/mol | Reaction Energy/kJ/mol | |
|---|---|---|
| Exo Reaction pathway | +119.7 | +20.6 |
| Endo Reaction pathway | +111.9 | +16.2 |
Table 14 and 15 show the results of the potential energy of reactants, TS and products; Activation energy and Reaction energy of this Diels-Alder reaction. Activation energy of this Diels Alder reaction is much higher than that of the reaction shown in Ex 3 ( refer to Table 13). Additionally, it is shown that both endothermic and exothermic reaction pathway of this Diels Alder reaction give a positive Gibbs Free energy. As a result, it can be concluded that the endo and exo Diels Alder reaction are very thermodynamically and kinetically unfavourable at this site.
Conclusion
In conclusion, various Diels Alder reaction and Cheletropic reaction pathways are analysed using computational method, Gaussian at PM6 and B3LYP method. At PES, minimum corresponds to reactants and products respectively. There is no imaginary frequency (negative frequency) in both reactants and products. TS corresponds to the first order saddle point which can be further confirmed by vibration calculation in Gaussian. This is confirmed by one negative frequency at TS. Overall, it is shown that computational experimental values are consistent with theoretical prediction.
In the Reaction of Butadiene with Ethylene, it is not very efficient due to the lack of electron donating group present in diene. In the reaction of Cyclohexadiene and 1,3-Dioxole, it is concluded that endothermic product is both kinetically and thermodynamically favourable. This can be explained by the secondary orbital interaction between the diene and substituent of dienophile. In contrast, exo reaction pathway is only favourable by sterics. Frontier Molecular Orbital analysis is also used to confirm the reaction is an inverse electron demand Diels Alder reaction. On the other hand, Diels alder reaction and Cheletropic reaction pathways are compared in the o-Xylylene-SO2 Cycloaddition. Despite the high activation barrier of Cheletropic pathways, it is thermodynamically favourable with the most negative Gibbs Free energy of the reaction. Additionally, a second cis-butadiene fragment in o-xylylene that can undergo a Diels-Alder reaction. However, it is proved that the endo and exo Diels Alder reaction are very thermodynamically and kinetically unstable with positive Gibbs free energy and high activation barrier.
As a result, computation calculation performed by Gaussian provide important insight to thermodynamic and kinetic parameter to Diels Alder reaction and Cheletropic reaction. More advance optimisation and frequency analysis can be completed in the future to provide a more accurate result in the future.
Log Files
Exercise 1
| Basics Set | Butadiene | Ethylene | Transition States | IRC | Product |
|---|---|---|---|---|---|
| PM6 | File:REACTANT1 2 OPTMIN.LOG | File:REACTANT2 OPTMIN.LOG | File:TS 2 OPTTS.LOG | File:TS 2 IRC out.log | File:P FINALOPT 2.LOG |
Exercise 2
| Basics Set | IRC | Exo-Products | Endo-Products |
|---|---|---|---|
| PM6 | File:Endo IRC output final.log File:EXO IRC 5.LOG |
File:Yts EXO P FINALOPTMIN 1.LOG | File:Yts ENDO P OPTMIN PM6.LOG |
| B3LYP/6-31G(d) | File:EXO P FINALOPTMIN B3LYP.LOG | File:Yts ENDO P OPTMIN B3LYP.LOG |
Exercise 3
| reaction | Xylylene | SO2 | IRC | Products |
|---|---|---|---|---|
| Exo | File:REACTANT 1 OPTMIN 3.LOG] | File:SO2 OPTMIN PM6.LOG | File:3 DA IRC opt.log | File:EX3 EXO P OPTMIN FINAL.LOG |
| Endo | File:ENDO IRC 6.LOG | File:ENDO P FINAL OPTMIN.LOG | ||
| Cheletropic | File:CHEL IRC.LOG | File:CHEL FINALP2.LOG |
Extra
| Reaction type | Transition States | Products |
|---|---|---|
| Exo | File:YTSTSOPTTS EXO.LOG | File:YTS EXO PRODUCT OPTMIN.LOG |
| Endo | File:YTSTSOPTTS ENDO.LOG | File:YTS ENDO PRODUCT OPTMIN.LOG |
References
- ↑ Lewars, E. Computational chemistry: introduction to the theory and applications of molecular and quantum mechanics; Springer: New York, 2011.
- ↑ Houston, P. L. Chemical kinetics and reaction dynamics; Dover: Mineola, NY, 2006.
- ↑ Bondi, A. The Journal of Physical Chemistry 1964, 68 (3), 441–451.
- ↑ Fox, M. A.; Whitesell, J. K. Organische Chemie: Grundlagen, Mechanismen, bioorganische Anwendungen; Spektrum Akadem. Verl.: Heidelberg, 1995.
- ↑ Chiang, J. F.; Bauer, S. H. Journal of the American Chemical Society 1969, 91 (8), 1898–1901.
- ↑ Fleming, I. Frontier orbitals and organic chemical reactions; Wiley: London, 2007.
- ↑ Alston, P. V.; Ottenbrite, R. M.; Cohen, T. The Journal of Organic Chemistry 1978, 43 (10), 1864–1867.