Rep:Mod:TS1111
Module 2
BH3 Optimisation
The result of the completed BH3 optimisation using the 3-21G basis set can be found below.
| File Name | bh3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -26.46226338 a.u. |
| RMS Gradient Norm | 0.00020672 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time | 27.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
The summary and item table show that the optimisation was completed succefully.

The graph shows the energy and gradient of the molecule at each stage of the optimisation. The gradient decreases as the energy decreases, with the gradient approaching 0 as the molecule is optimised. A successful optimisation should have a gradient of less than 0.001.
What is a gradient?
At the first stage of the optimisation Gaussview shows a molecule with no bonds this is because Gaussview is programmed with a strict definition of what a bond is, sometimes if the bond length is too long and does not fit the distance criteria, gaussview does not show the bond as being present. This does not mean that there is no bond, only that the bond does not conform to the rules set by gaussview.
In some structures gaussview does not draw in the bonds where we expect, does this mean there is no bond? Why? What is a bond?
The optimization plot shows the gradient decreasing as the energy decreases, reaching 0 when the molecule is optimized. The gradient of the Energy plot shows the force on the molecule. When the gradient is 0 there is no net force on the molecule and it is therefore in equilibrium.
BH3 Optimisation using a better basis set
The BH3 Optimisation was then re-run using the 6-31G(d,p) basis set.
File:BH3 OPT 631G DP ts3610.LOG
| File Name | bh3_opt_613g_dp |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -26.61532264 a.u. |
| RMS Gradient Norm | 0.00021663 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | CS |
| Job cpu time | 1 minutes 16.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000434 0.000450 YES
RMS Force 0.000284 0.000300 YES
Maximum Displacement 0.001698 0.001800 YES
RMS Displacement 0.001114 0.001200 YES
Predicted change in Energy=-1.188111D-06
Optimization completed.
-- Stationary point found.
The summary and item table show that the molecule has been optimised successfully.
| B-H bond distance | 1.19347 |
| H-B-H bond angle | 120.00 |
Total energy for 3-21G optimised structure: -26.46226338 a.u.
Total energy of 6-31G (d,p) optimised structure: -26.61532264 a.u.
The energies for the two optimised structures cannot be directly compared as different basis sets were used.
TlBr3 Optimisation
The TlBr3 optimisation was carried out using the LANL2DZ basis set, the results can be seen below.
TlBr3 Optimisation DOI:10042/20433
| File Name | tlbr3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -91.21812851 a.u. |
| RMS Gradient Norm | 0.00000090 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time | 18.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-6.084033D-11
Optimization completed.
-- Stationary point found.
The summary and item table show that the molecule has been optimised successfully.
| Tl-Br bond distance | 2.65095Å |
| Br-Tl-Br bond angle | 120.00° |
Tl-Br bond distance literature value comparison.
The literature value for the Tl-Br bond distance was found to be 2.52Å[1]. This is shorter than the bond distance that was produced by Gaussian. This is probably because pseudo potentials were used in the optimisation.
BBr3 Optimisation
| File Name | bbr3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -64.43645277 a.u. |
| RMS Gradient Norm | 0.00000384 a.u. |
| Dipole Moment | 0.0002 Debye |
| Point Group | CS |
| Job cpu time | 35.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000037 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.109337D-10
Optimization completed.
-- Stationary point found.
The summary and item table show that the molecule was successfully optimised.
| B-Br bond distance | 1.93397 |
| Br-B-Br bond angle | 120.002 |
| B-H | 1.19347 |
| Tl-Br | 2.65095 |
| B-Br | 1.93397 |
BH3 has the shortest bond length with TlBr3 having the longest. Changing the ligand from Hydrogen to Bromine increases the bond length as can be seen by the difference in the B-H and B-Br bond lengths recorded. This is because Bromine is a much larger molecule than Hydrogen and has more diffuse valence orbitals. The Hydrogen and Boron orbitals are closer in size than Boron and Bromine meaning there will be a better orbital overlap and s stronger bond will be formed, which in turn will also be shorter. Hydrogen and Boron also have similar electronegativities (2.20 and 2.04 respectively) which again suggests a strong, short bond.The overlap between the Boron and Bromine orbitals is much weaker and therefore produces a weaker bond. There is also a much greater difference in the electronegativities (0.92 as opposed to 0.16) which contributes to the bond being weaker and also longer. Both Hydrogen and Bromine are one electron donors, in the case of Hydrogen this comes from the 1s orbital whereas in Bromine it is donated from the 3p orbital.
Changing the central atom from Boron to Thallium also increases the bond length, as can be seen in the difference between B-Br and Tl-Br. This is again because Thallium is a much larger molecule than Boron. Thallium and Boron are both in group 3 of the periodic table therefore they have the same number of valence electrons, however because Thallium is a much larger atom the orbitals are more diffuse and therefore the overlap is not as good. This means that the bond is weaker and hence has a greater bond distance.
BH3 Frequency
File:TONISEMMENCE BH3 FREQ.LOG
A frequency analysis is carried out to ensure that the stationary point found during the optimisation calculation is a minimum.
The first set of data for the low frequencies shows the movement of the centre of mass of the molecule, this is ideally as small as possible and they can be used to determine whether an appropriate basis set was used. The second set of data shows the vibrational frequencies of the molecule, for a molecule that has been correctly optimised to a minimum all of these values should be positive.
Low frequencies --- -73.0837 -69.5094 -69.4394 -0.0007 -0.0003 0.0005
Low frequencies --- 1161.3810 1212.0964 1212.1646
The frequencies are not within +/- 12 as they should be for a good calculation, this is because the basis set used was not suitable for the molecule. The are no negative frequencies.
It is important that the same basis set is used for both the optimisation and frequency calculations, because different basis sets will optimise the molecules in different ways and to different values. For the frequency calculation to be valid it must be completed using the same basis set as the optimisation. Calculations that use different basis sets cannot be directly compared to one another.
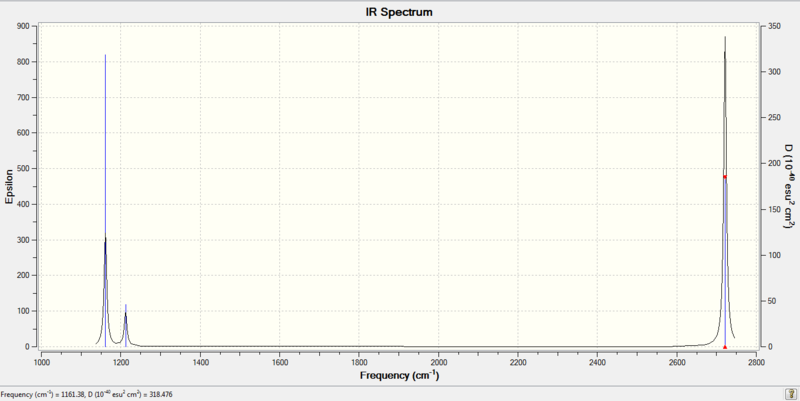
IR spectrum of BH3
It is clear that there are 6 separate vibrations, however only 3 peaks appear on the IR spectrum. Vibrations 2 and 3 are degenerate therefore they only produce 1 peak on IR spectrum at 1212 cm-1, vibrations 5 and 6 are also degenerate so for the same reason only one peak is seen at 2721 cm-1. Vibration 4 is totally symmetric, therefore there is no net dipole moment and this vibration does not show up on the IR spectrum. The 3rd peak is attributed to vibration 1 at 1161 cm-1.
TlBr3 Analysis
When I first ran the frequency calculation, I computed it using the same basis set as I had for BH3 which was 6-31G (d,p). Below is the file for the calculation.
TlBr3 Frequency (failed) DOI:10042/20437
The calculation did not run to completion and the below error was recorded.
Atomic number out of range for 6-31G basis set.
I realised that this is because Tl is such a large element and the basis set was not suitable. The calculation was re-run using a more appropriate basis set, LANL2DZ, and the correct file can be seen below.
TlBr3 Frequency DOI:10042/20478
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9367 3.9367
Low frequencies --- 46.4289 46.4292 52.1449
The frequencies are within +/-12 which shows the calculation was completed under suitable condition and there are no negative frequencies present.
IR Spectrum of TlBr3
The IR spectrum again shows only 3 peaks whcih is to be expected as both BH3 and TlBr3 have the D3h point group.
| no. | BH3 Frequency | TlBr3 Frequency |
|---|---|---|
| 1 | 1161.38 | 46.43 |
| 2 | 1212.10 | 46.43 |
| 3 | 1212.16 | 52.14 |
| 4 | 2587.81 | 165.27 |
| 5 | 2721.51 | 210.69 |
| 6 | 2721.52 | 210.69 |
As previously mentioned, both IR spectra show 3 peaks which is expected for the D3h point group. The large difference in frequencies between BH3 and TlBr3 is due to the large difference in mass between the 2 molecules. TlBr3 is much heavier and therefore the vibrational frequencies are much lower. In the IR spectrum of BH3 the wagging motion has the lowest vibrational frequency, whereas in the TlBr3 spectrum it is the 3rd frequency. This is again due to the fact that TlBr3 is a much heavier molecule.
The A2 and E' modes and the A1' and E' modes lie close together because the A2 and E' modes are both bending modes and therefore lie at a lower frequency than the stretching modes of A1' and E'.
Molecular Orbitals
BH3 Population Analysis DOI:10042/20442
MO Diagram
There appears to be no real significant difference between the real and LCAO MO's. The main difference is that the real orbitals overlap more and merge together to appear as one, whereas the LCAO molecular orbitals show no substantial overlap. Qualitative MO theory appears to be quite accurate in determining the shape of the orbitals but cannot take into account the size or overlap. It is a useful method to predict the shape of the real MO's.
NBO Analysis
The NH3 optimisation was carried out using the 6-31G basis set.
NH3 Optimisation DOI:10042/20445
| File Name | logfile_nh3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -56.54794765 a.u. |
| RMS Gradient Norm | 0.00001026 a.u. |
| Dipole Moment | 1.0122 Debye |
| Point Group | C3V |
| Job cpu time | 17.3 seconds |
Item Value Threshold Converged?
Maximum Force 0.000019 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000042 0.001800 YES
RMS Displacement 0.000028 0.001200 YES
Predicted change in Energy=-1.075064D-09
Optimization completed.
-- Stationary point found.
The summary and item table show that the molecule was optimised successfully.
Low frequencies --- -22.3657 -22.3456 -0.0046 0.0243 0.0630 19.1934
Low frequencies --- 1132.0960 1726.9861 1726.9864
There are no negative frequencies.
NH3 Population Analysis DOI:10042/20462
Charge limits: -1.109 to 1.109
It is clear to see that most of the electron density lies on the Nitrogen atom which is expected for such a highly electronegative atom, the Nitrogen atom also has a lone pair of electrons which contributes to the high amount of electron density.
Association energies: Ammonia-Borane
Ammonia-Borane Optmisation DOI:10042/20465
| File Name | nh3bh3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -83.22469002 a.u. |
| RMS Gradient Norm | 0.00006847 a.u. |
| Dipole Moment | 5.5654 Debye |
| Point Group | C1 |
| Job cpu time | 1 minutes 30.4 seconds |
Item Value Threshold Converged?
Maximum Force 0.000139 0.000450 YES
RMS Force 0.000063 0.000300 YES
Maximum Displacement 0.000761 0.001800 YES
RMS Displacement 0.000339 0.001200 YES
Predicted change in Energy=-2.028975D-07
Optimization completed.
-- Stationary point found.
Ammonia-Borane Frequency DOI:10042/20466
Low frequencies --- -0.0011 -0.0009 0.0008 19.0738 23.7371 42.9797
Low frequencies --- 266.5931 632.3886 639.5045
The low frequencies are out of the normal acceptable range but this was expected due to the basis sets not being entirely suitable for a larger molecule.
| E(NH3) | -56.54794765 a.u |
| E(BH3) | -26.61532264 a.u |
| E(NH3NH3) | -83.22469002 a.u |
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.06141973 a.u
Dissociation energy: -161.3 kj mol-1 (4s.f.)
Ionic Liquids: Designer Solvents
Comparison of selected 'onium' cations
[N(CH3)4]+
The [N(CH3)4]+ optimisation and frequency analysis were carried out using the 6-31G basis set.
N(CH3)4+ Optimisation and Frequency DOI:10042/20554
| File Name | [N(CH3)4]+ opt + freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| E(RB3LYP) | -214.18127254 a.u. |
| RMS Gradient Norm | 0.00002015 a.u. |
| Dipole Moment | 5.7238 Debye |
| Point Group | C1 |
| Job cpu time | 7 minutes 54.8 seconds |
Item Value Threshold Converged?
Maximum Force 0.000074 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000483 0.001800 YES
RMS Displacement 0.000184 0.001200 YES
Predicted change in Energy=-4.326677D-08
Optimization completed.
-- Stationary point found.
The summary and item table show that the molecule was optimised successfully.
Low frequencies --- -14.7279 -4.8540 0.0003 0.0008 0.0011 8.3232
Low frequencies --- 179.2379 278.4415 285.2962
The frequencies are within the +/-15 range and there are no negative frequencies.
N(CH3)4+ Population Analysis DOI:10042/20574
 |
 |
 |
 |

|
| 6 | 10 | 14 | 18 | 21 |
MO 6 is the first valence molecular orbital and it shows strong bonding character with a lot of delocalisation. MO 10 shows strong out of phase interactions with some weak through space interactions, the delocalisation is limited. MO 14 has 2 nodes, it is similar to the dz2 and shows a lareg amount of delocalisation, again there is strong out of phase interactions with some weak through space interactions. MO 18 has strong out of phase interactions and some weak through space in phase interactions. There is little delocalisation. MO 21 is the HOMO, it has 2 nodes and strong out of phasen interactions. The through space interactions can be considered weak. There is some delocalisation around the CH3 groups.
in your wiki present 5 MOs (from [N(CH3)4]+) ranging from highly bonding to highly antibonding (from any of the MOs you have visualised) and describe the interactions occuring in the MOs (for example which AOs have strong/weak bonding/antibonding overlap? Are there through space interactions? How many and what kinds of nodes are there? How delocalised is the MO?)
Charge range: -0.483 to 0.483
From the NBO analysis it can be seen that there is more electron density on the Carbon atoms than on the Nitrogen atom. This suggests that depicting the positive charge on the Nitrogen (as shown below) is not unreasonable as it would explain why there is electron density on the Nitrogen atom than would normally be expected for a highly electronegative atom.
[P(CH3)4]+
The [P(CH3)4]+ optimisation and frequency calculations were carried out using the 6-31G basis set.
P(CH3)4+ Optimisation and Frequency DOI:10042/20555
| File Name | [P(CH3)4]+ opt +freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| E(RB3LYP) | -500.82700252 a.u. |
| RMS Gradient Norm | 0.00003915 a.u. |
| Dipole Moment | 3.7945 Debye |
| Point Group | C1 |
| Job cpu time | 7 minutes 39.5 seconds |
Item Value Threshold Converged?
Maximum Force 0.000144 0.000450 YES
RMS Force 0.000025 0.000300 YES
Maximum Displacement 0.001031 0.001800 YES
RMS Displacement 0.000291 0.001200 YES
Predicted change in Energy=-1.294596D-07
Optimization completed.
-- Stationary point found.
The summary and item table show the molecule was optimised successfully.
Low frequencies --- -20.1133 -9.6973 -0.0030 -0.0011 0.0005 11.7895
Low frequencies --- 151.7197 182.0758 189.6499
The frequencies are only just outside the +/-15 range, but within the +/-30 range which suggests that the basis set used was not entirely suitable as there are some soft vibrational modes. There are no negative frequencies.
P(CH3)4+ Population Analysis DOI:10042/20575
Charge Range: -1.667 to 1.667
The Phosphorus atom has the least electron density which would suggest that it carries the formal positive charge.
[S(CH3)3]+
The [S(CH3)3]+ optimisation and frequency calculations were carried out using the 6-31G basis set.
S(CH3)3+ Optimisation and Frequency DOI:10042/20561
| File Name | [S(CH3)3]+ opt + freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| E(RB3LYP) | -517.68327910 a.u. |
| RMS Gradient Norm | 0.00001039 a.u. |
| Dipole Moment | 3.1198 Debye |
| Point Group | C1 |
| Job cpu time | 3 minutes 43.8 seconds |
Item Value Threshold Converged?
Maximum Force 0.000040 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.000519 0.001800 YES
RMS Displacement 0.000142 0.001200 YES
Predicted change in Energy=-1.109381D-08
Optimization completed.
-- Stationary point found.
The summary and item table show that the molecule was optimised successfully.
Low frequencies --- -12.9920 -8.1331 -0.0034 -0.0020 0.0032 22.9236
Low frequencies --- 158.4638 194.1190 198.5758
The frequencies are only slightly outside the +/-15 acceptable range, but still within the +/-30 range. There are no negative frequencies.
S(CH3)3+ Population Analysis DOI:10042/20576
Charge Range: -0.917 to 0.917
The Sulphur atom again carries the least electron density which suggests that this is where the positive charge is situated. The axial and equatorial Hydrogen atoms have different values for the charge distribution. (Heq=0.297, Hax=0.279) this is probably due to different through space interactions.
NBO Analysis Comparison
| Bond | Charge on C atom | Charge on X atom | C Contribution | X Contribution |
|---|---|---|---|---|
| C-N | -0.483 | -0.295 | 33.65% | 66.35% |
| C-P | -1.060 | 1.667 | 59.58% | 40.42% |
| C-S | -0.845 | 0.917 | 48.67% | 51.33% |
Nitrogen is the most electronegative so is expected to have the most electron density situated on it compared to Phosphorus or Sulphur. Sulphur is more electronegative than Phosphorus so has a less positive value in the charge distribution. The charge on the Hydrogen atoms in all 3 cations is quite similar because the Carbon charges change with the X atom meaning the Hydrogen atoms are much less affected.
The relative contributions to the C-X bond can also be explained by the electronegatvities. Nitrogen is the most electronegative and therefore has the most electron density to contribute to the C-X bond. Sulphur is the second most electronegative and hence contributes less to the C-X bond than Nitrogen does, but more so that Phosphorus. Sulphur and Carbon have similar electronegativies and therefore contribute almost equal amounts to the C-X bond.
Influence of functional groups
[N(CH3)3(CH2OH)]+
The [N(CH3)3(CH2OH)]+ optimisation and frequency calculations were completed using the 6-31G basis set.
N(CH3)3(CH2OH)+ Optimisation and Frequency DOI:10042/20601
| File Name | [N(CH3)3(CH2OH)]+ opt+freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| E(RB3LYP) | -289.39322240 a.u. |
| RMS Gradient Norm | 0.00002706 a.u. |
| Dipole Moment | 6.5024 Debye |
| Point Group | C1 |
| Job cpu time | 10 minutes 16.2 seconds |
Item Value Threshold Converged?
Maximum Force 0.000053 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000530 0.001800 YES
RMS Displacement 0.000117 0.001200 YES
Predicted change in Energy=-4.377935D-08
Optimization completed.
-- Stationary point found.
The summary and item table show that the optimisation was completed successfully.
Low frequencies --- -111.3678 -17.6168 -0.0007 -0.0004 0.0005 4.3486
Low frequencies --- 12.0746 128.3809 213.0549
One of the frequencies is very far out of the acceptable range. None of the frequencies are negative.
N(CH3)3(CH2OH)+ Population Analysis DOI:10042/20609
Charge Range:-0.757 to 0.757
[N(CH3)3(CH2CN)]+
The [N(CH3)3(CH2CN)]+ optimisation and frequency calculations were carried out using the 6-31G basis set.
N(CH3)3(CH2CN)+ Optimisation and Frequency DOI:10042/20603
| File Name | [N(CH3)3(CH2CN)]+ opt+freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | singlet |
| E(RB3LYP) | -306.39376954 a.u. |
| RMS Gradient Norm | 0.00002229 a.u. |
| Dipole Moment | 3.5229 Debye |
| Point Group | C1 |
| Job cpu time | 10 minutes 51.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000058 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.001595 0.001800 YES
RMS Displacement 0.000503 0.001200 YES
Predicted change in Energy=-7.361346D-08
Optimization completed.
-- Stationary point found.
The summary and item table show that molecule was optimised successfully.
Low frequencies --- -13.7129 0.0010 0.0011 0.0012 9.7360 10.9250
Low frequencies --- 89.4615 154.5353 204.7819
N(CH3)3(CH2CN)+ Population Analysis DOI:10042/20612
Charge Range: -0.489 to 0.489
MO Analysis and Comparison
OH is an electron withdrawing group so the majority of the charge lies on the Oxygen atom, with very little on the adjacent Carbon atom. The Hydrogen adjacent to the Oxygen atom has a high positive chareg associated with it due to the electronegativity of the Oxygen. CN is an electron donating group and because of this most of the electron density is now situated on the Carbon atoms. The Carbon of the CN group has a positive charge due to the electronegativity of the Nitrogen atom.
| [N(CH3)4]+ | [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ | |
| HOMO |  |
 |
 |
| LUMO |  |
 |
 |
The HOMO of [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+ appear to be quite similar, there is very little delocalisation across the molecule with most of the electron density being on the -OH and -CN groups respectively. The HOMO of [N(CH3)4]+ has 2 nodes and shows a large amount of delocalisaton over the whole molecule.
The LUMO's are all quite similar with the [N(CH3)4]+ LUMO showing the most delocalisation and the [N(CH3)3(CH2OH)]+ LUMO showing the least delocalisation.
The -OH causes both the HOMO and LUMO energy to be raised, but the HOMO-LUMO gap is reduced. This suggests that the molecule will be less reactive than [N(CH3)4]+. The addition of a -CN group also causes the HOMO-LUMO gap to be reduced as the energy of the HOMO is increased whilst teh energy of the LUMO is decreased. this suggests that the molecule will be more reactive than [N(CH3)4]+.
References
<references> [1]
- ↑ 1.0 1.1 Blixit J, Glaser J, Mink J, Persson I,Persson P, Sandstroem M; Structure of Thallium(III) Chloride, Bromide, and Cyanide Complexes in Aqueous Solution, J. Am. Chem. Soc., 1995, 117 (18), pp 5089–5104. DOI:10.1021/ja00123a011