Rep:Mod:TPD17Inorganic
EX3 Section
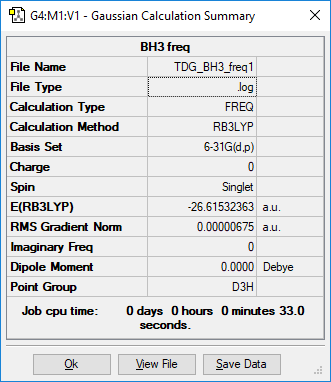
BH3
identify the method:RB3LYP basis set: 6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000043 0.001800 YES RMS Displacement 0.000028 0.001200 YES
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
Borohydrate |
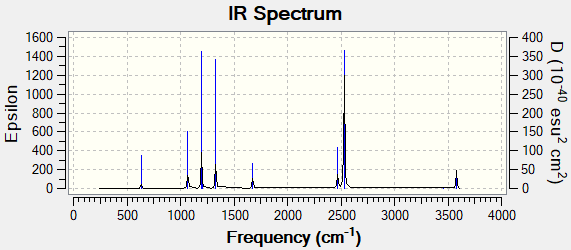
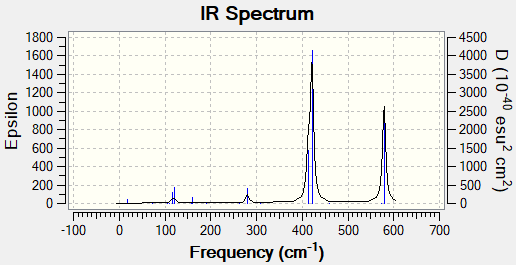
Vibrational spectrum for NH3
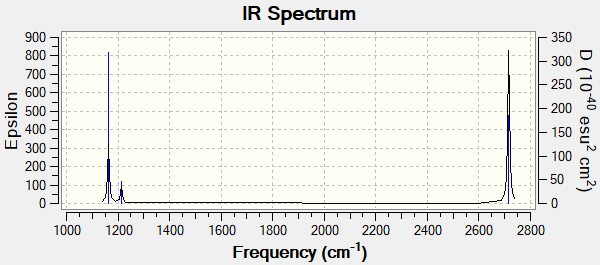
| wavenumber (cm-1 | Intensity (arbitrary units) | IR active? | type |
| 1163 | 93 | yes | out-of-plane bend |
| 1213 | 14 | slightly | bend |
| 1213 | 14 | slightly | bend |
| 2582 | 0 | no | symmetric stretch |
| 2716 | 126 | yes | asymmetric stretch |
| 2716 | 126 | yes | asymmetric stretch |
There are three peaks in the spectrum for BH3 because the symmetric stretch is not IR active as there is no change in dipole. The other two missing peaks are in the same place as their degenerate vibrations. There are two peaks at 1213 cm-1 and at 2716 cm-1.
Ng611 (talk) 23:40, 15 May 2019 (BST) Good!
There are no real significant differences between real and LCAO MO's. The higher energy antibonding orbitals seem to fit the LCAO MO's less well, perhaps due to other orbitals that were not included in the qualitative MO diagram, like the hydrogen p orbitals.
This suggests that qualitative MO theory is a useful and relatively accurate tool to find the overall shapes and positions of MO's, especially the filled ones.
Ng611 (talk) 23:40, 15 May 2019 (BST) Can you be more specific about how the antibonding MOs fit 'less well'. You are correct that there are some discrepancies, but you need to be more specific in your answer to get the credit.
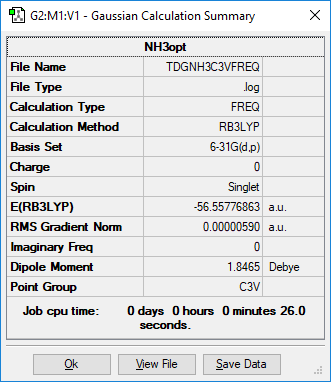
NH3
identify the method:RB3LYP basis set: 6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000040 0.001800 YES
RMS Displacement 0.000013 0.001200 YES
Low frequencies --- -8.5223 -8.4750 -0.0029 0.0335 0.1918 26.4067 Low frequencies --- 1089.7616 1694.1862 1694.1866
Ammonia |
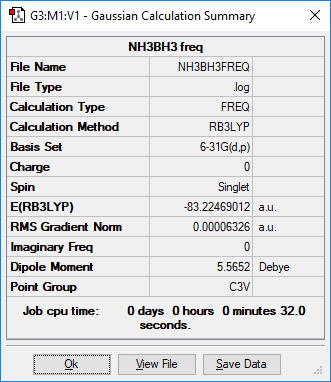
NH3BH3
identify the method:RB3LYP basis set: 6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000114 0.000450 YES
RMS Force 0.000063 0.000300 YES
Maximum Displacement 0.000621 0.001800 YES
RMS Displacement 0.000355 0.001200 YES
Low frequencies --- -0.0617 -0.0457 -0.0065 21.6788 21.6847 40.5418 Low frequencies --- 266.0172 632.3610 640.1362
Ammonia borane |
E(NH3)= -56.558
E(BH3)= -26.615
E(NH3BH3)= -83.225
then report the association energy first in AU and then converted to kJ/mol ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] -0.052 a.u. -136 kJ/mol
This is a sensible number as it is on the order of 102 kJ/mol
Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion? This is quite a weak bond compared to a c-c single bond that is around 347 kJ/mol.
Ng611 (talk) 23:43, 15 May 2019 (BST) You're so close to the right answer (about 1 kJ/mol off), which makes me think that it's because you rounded too early. Generally, you round to the 5th DP when working in Hartrees, and then round to the nearest kJ/mol when you've arrived at your final answer.
N3
identify the method:RB3LYP basis set: Gen
Item Value Threshold Converged?
Maximum Force 0.000088 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.000858 0.001800 YES
RMS Displacement 0.000481 0.001200 YES
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
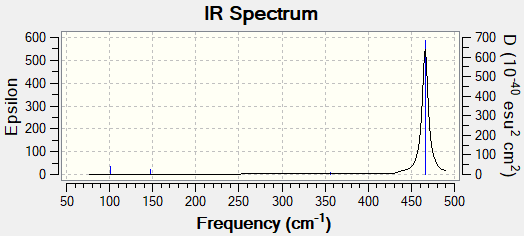
nitrgen Iodide |
N-I bond length
The N-I bond is 2.184 Angstroms
Project Section
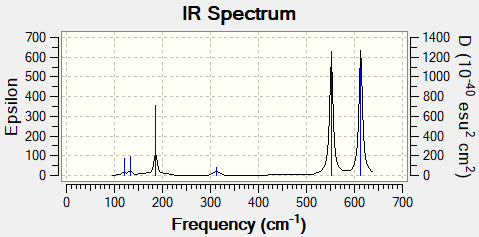
Al2Cl4Br2
Bridging Isomer
identify the method:RB3LYP basis set: GEN
Ng611 (talk) 23:46, 15 May 2019 (BST) GEN is not a valid basis set descriptor. You should write it as 6-31G(d,p)/LANL2DZ
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000687 0.001800 YES
RMS Displacement 0.000319 0.001200 YES
Low frequencies --- -4.8281 -4.7248 -2.9327 -0.0011 0.0010 0.0012 Low frequencies --- 14.9942 63.3011 86.0723
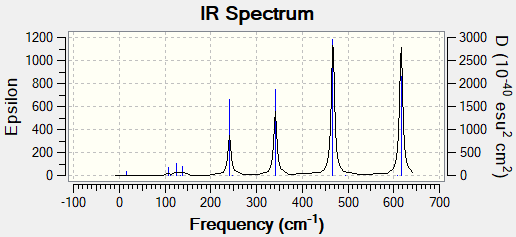
Br bridging isomer |
trans Isomer
identify the method:RB3LYP basis set: GEN
Item Value Threshold Converged?
Maximum Force 0.000040 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000649 0.001800 YES
RMS Displacement 0.000272 0.001200 YES
Low frequencies --- -4.0622 -2.1902 -0.0028 -0.0025 0.0015 1.0660 Low frequencies --- 17.7403 48.9735 72.9546
Trans Isomer |
Energy
Energy of isomer with Br bridging ions -2352.406 Energy of isomer with trans terminal Br ions -2352.416
The trans isomer is more stable as it is 26 kJ/mol (0.01 a.u.) lower in energy.
Ng611 (talk) 23:49, 15 May 2019 (BST) Can you rationalise why this is?
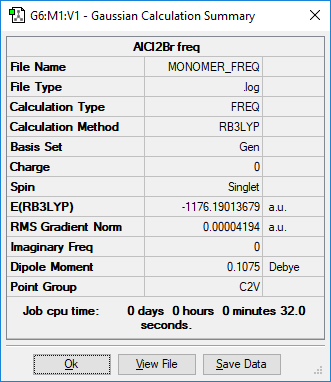
AlCl2Br
identify the method:RB3LYP basis set: GEN
Item Value Threshold Converged?
Maximum Force 0.000081 0.000450 YES
RMS Force 0.000042 0.000300 YES
Maximum Displacement 0.001588 0.001800 YES
RMS Displacement 0.000974 0.001200 YES
Low frequencies --- 0.0047 0.0051 0.0055 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
AlCl2Br |
Dissociation Energy
The dissociation energy of the more stable conformer, the one with trans terminal Br ions to AlCl2Br:
Delta E=2E(AlCl2Br)-E(trans Al2Cl4Br2)
2*(-1176.190)-(-2352.416)=0.036 a.u. = 95 kJ/mol
This is a positive energy meaning that forming two monomers from a dimer is unfavourable. So the dimer product is more stable than the isolated monomers.
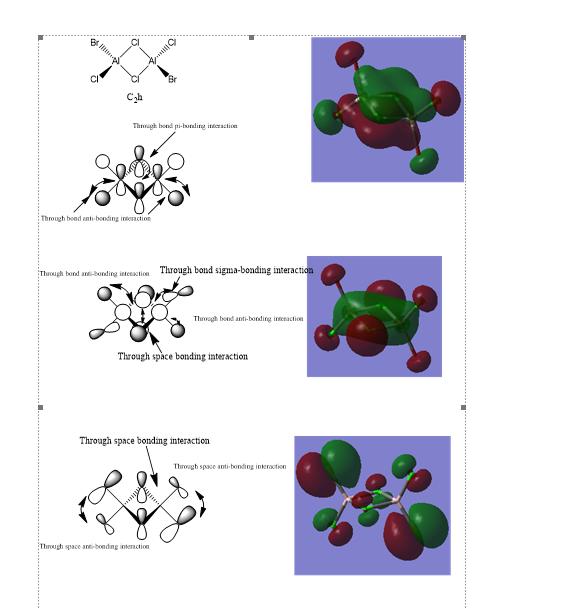
Molecular orbitals
Ng611 (talk) 23:53, 15 May 2019 (BST) More detail is needed in a few of these. Most importantly, are these orbitals bonding, antibonding, or non-bonding?