Rep:Mod:TKZ2018
BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000018 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.000105 0.001800 YES RMS Displacement 0.000066 0.001200 YES
Frequency analysis log file: TK_BH3_FREQ.LOG
Low frequencies --- -0.6650 -0.3763 -0.0054 13.1509 16.6691 16.6824 Low frequencies --- 1163.0412 1213.2129 1213.2156
borane |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1126 | 146 | A1' | yes | out-of-plane bend |
| 1213 | 14 | E' | yes | bend |
| 1213 | 14 | E' | yes | bend |
| 2582 | 0 | A2 | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
There are 5 IR active modes for borane molecules. 3 peaks can be observed on the provided IR spectrum. the peak at 1163 cm-1 contains 2 degenerate out-of-plane bends and the peak at 2715 cm-1 contains 2 degenerate asymmetric stretches and that is why 3 peaks are observed instead of 5.
MO diagram
The interaction between the valence orbitals of boron and the symmetry adapted orbitals for an H3 fragment are shown in this diagram. The 2 s orbital of boron has the same symmetry as the a1' orbital of H3, and are of similar energy. Therefore they interact favorably and the energy is lowered a fair bit. They form a pair of bonding/anti-bonding orbitals with a1' symmetry under the D3h group. The px and py orbitals are of the same symmetry as e' orbitals of H3 fragment. They also interact quite favorably and form two pairs of degenerate bonding/anti-bonding orbitals. The pz orbital remains non-bonding as it does not have the same symmetry as any other orbital. Besides the MO diagram sketch, the MOs calculated and visualized by Gaussian are shown.
Ng611 (talk) 20:59, 20 May 2018 (BST) Good MO analysis, well done. You also should comment on how similar the computed MOs are to those determined from qualitative MO theory.
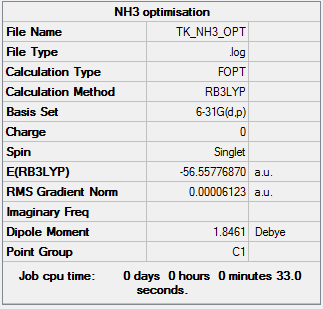
NH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000100 0.000450 YES RMS Force 0.000050 0.000300 YES Maximum Displacement 0.000177 0.001800 YES RMS Displacement 0.000113 0.001200 YES
Frequency analysis log file: TK_NH3_FREQ.LOG
Low frequencies --- -41.0309 -10.5736 -0.0011 -0.0004 0.0015 19.6249 Low frequencies --- 1089.0806 1693.8797 1694.2403
ammonia |
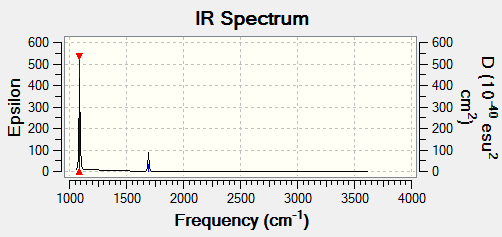
Vibrational spectrum for NH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1089 | 145 | A1 | yes | out-of-plane bend |
| 1694 | 14 | E | yes | bend |
| 1694 | 14 | E | yes | bend |
| 3462 | 1 | A1 | no | symmetric stretch |
| 3591 | 0 | E | no | asymmetric stretch |
| 3591 | 0 | E | no | asymmetric stretch |
There are 3 IR active modes for ammonia molecules. 2 peaks can be observed on the provided IR spectrum. the peak at 1694 cm-1 contains 2 degenerate bends and that is why 2 peaks are observed instead of 3.
NH3-BH3 association energy
E(NH3)= -26.61532 E(BH3)= -56.55777 E(NH3BH3)= -83.22469
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -135.476 kJ/mol
Ng611 (talk) 21:01, 20 May 2018 (BST) The final value should be reported to the nearest kj/mol. Also, how strong is this bond? A comparison with other bond energies would be useful.
BBr3
B3LYP/6-31G(d,p) for B, B3LYP/LanL2DZ for Br
Link to the published calculation in D-Space: DOI:10042/202378
Item Value Threshold Converged? Maximum Force 0.000023 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.000130 0.001800 YES RMS Displacement 0.000069 0.001200 YES
Frequency analysis log file: TK_BBR3_FREQUENCY.LOG
Low frequencies --- -3.0713 -0.0002 -0.0001 0.0001 2.2003 3.6050 Low frequencies --- 155.9034 155.9851 267.7063
BBr |
Vibrational spectrum for BBr3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 156 | 0 | A2 | no | in-plane bend |
| 156 | 0 | A1' | no | in-plane bend |
| 268 | 0 | E' | no | symmetric stretch |
| 378 | 4 | A1' | very slightly | out-of-plane bend |
| 763 | 320 | E' | yes | asymmetric stretch |
| 763 | 320 | E' | yes | asymmetric stretch |
There are 2 IR active modes for BBr3 molecules. 1 peak can be observed on the provided IR spectrum. The peak at 763 cm-1 contains 2 degenerate stretches and that is why 1 peak is observed instead of 2.