Rep:Mod:TK2814
Introduction
In this experiment, the transition structures of three Diels-Alder reactions and one Cheletropic reaction were modelled, calculated and compared by using Gaussview 5.0 via different optimization methods. The structures of the transition states and the activation energies were investigated to support the preferred pathways of the reactions.
Reaction of Butadiene with Ethylene
The reaction between 1,3-butadiene with 4π electrons and ethylene with 2π electrons is described as a [4+2] cycloaddition reaction.

Optimization
1,3-butadiene and ethylene were constructed in the Gaussview 5.0 and optimized at PM6 level. The optimised reactants were joined together to construct the transition state, which was also optimized at PM6 level. The cyclohexene was construct and optimized at PM6 level as well.
Analysis
The MO diagram for the formation of the butadiene and ethlyene is shown below, where the HOMO of butadiene is ungerade, the LUMO of butadiene is gerade, the HOMO of ethlyene is gerade and the LUMO of ethlyene is ungerade.
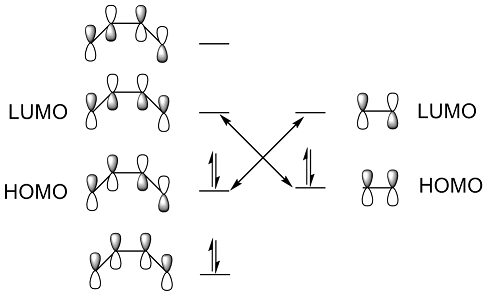
The HOMOs and LUMOs of butadiene, ethlyene and TS were visualized.
Nf710 (talk) 09:27, 4 November 2016 (UTC) You havent given an MO diagram of the products.
| 1,3-butadiene | ethylene | transition state | |
|---|---|---|---|
| HOMO | |||
| LUMO |
The HOMO of butadiene shows characteristic of anti-bonding. The LUMO of ethylene is anti-bonded. The ungerade- ungerade interaction results an anti-bonding orbital of the TS. The LUMO of butadiene is a bonding orbital. The HOMO of ethylene also shows a strong bonding effect, the gerade- gerade interaction results a strong bonded orbital in the TS.
In general, the length of C-C double bonds were shorter than that of single bonds. The length of C-C double bond decreased, the single bond increased during the reaction. The length C-C double bonds of reactant were 1.379A and the the length C-C single bond was 1.410A. The bond length remained the same at TS. The C-C double bond decreased to 1.33A and the single bonds increased to 1.49A and 1.53A for different bonds after the reaction.

At the imaginary frequency, the transition state
is more like symmetricly stretching. The atoms that we expect to form bonds between them are getting closer to each other, which is good for the reaction to take place. Because of the symmetric stretch, the formation of the bonds is synchronous. However, at the lowest positive frequency, the molecules are twisting, they bend. The uneven movement of the atoms might cause the formation of the bonds asynchronous
Nf710 (talk) 09:33, 4 November 2016 (UTC) Good underdstanding of frequency analysis but no mention of bond lengths in TS
Reaction of Benzoquinone with Cyclopentadiene
Optimization
The Benzoquinone and cyclopentadiene were constructed, optimized at PM6 level and refined at the B3LYP/6-31G (d) level. The optimised molecules were joined and adjusted to the endo TS form and the exo TS form. The bonds that would formed in the reaction were frozen. The two TS were optimised at the B3LYP/6-31G (d) level,
Analysis
| Endo | Exo | |
|---|---|---|
| HOMO | ||
| LUMO |
From the MO above we can see this is an inverse demand DA reaction.
The secondary orbital interactions are the interactions between non-bonding orbitals. The exo-product is more thermodynamically stable. However, as we can see above, the are lots of anti-nonding charactors in the HOMO of endo-transition state, the activation energy is therefore lowered. The endo-product is more kinetically favorable and it is the major product.
(It's hard to see what's going on with the MO diagrams you've included. You've said the exo product is more thermodynamically stable. However this isn't the case and you haven't included any data to back up your claims. However it is true that the endo TS is lower in energy due to SOO Tam10 (talk) 15:04, 27 October 2016 (BST))
Nf710 (talk) 09:45, 4 November 2016 (UTC) You have mentioned some reasoning to why the endo is lower in energy but the correct way to define this is by says secondary orbital interaction. Also it appears your MOs arn't optimised. You should have provided a log file in a Jmol so we could check your results.
Diels-Alder vs Cheletropic
Optimization
The reactants, transition states and products for both the Diels-Alder and Cheletropic reactions were optimized at the PM6 level.
(The reactants should ideally be calculated together to include their (favourable) interactions Tam10 (talk) 15:16, 27 October 2016 (BST))
Analysis
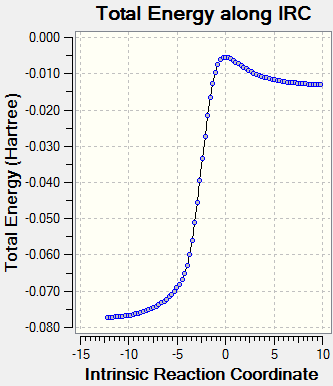
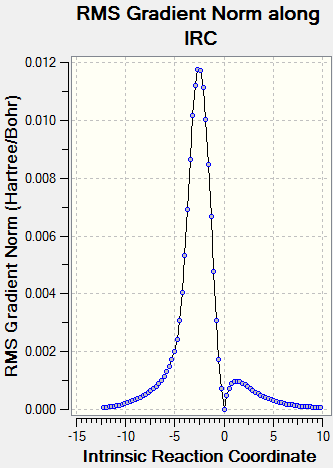
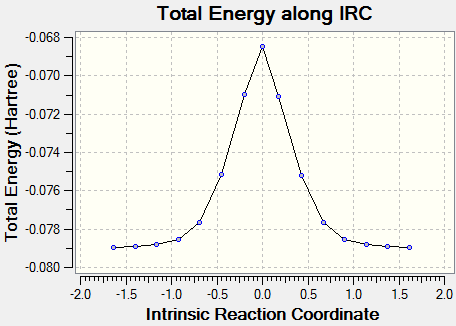
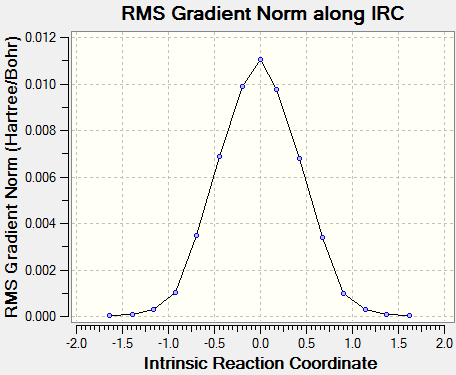
(The second IRC has failed. It fails in this way due to not being at the correct TS for a particular method/basis set - the gradient must be 0 at IRC=0 Tam10 (talk) 15:16, 27 October 2016 (BST))
| Sum of electronic and thermal Free Energies(Hartree/Particle) | |
|---|---|
Xylylene |
0.178746 |
| SO2 | -0.118614 |
| Endo-TS | 0.031779 |
| Exo-TS | 0.090559 |
| Cheletropic | 0.054916 |
(Some of these values are off, and you haven't included any files to see what happened. You also haven't included products. Tam10 (talk) 15:16, 27 October 2016 (BST))
Activation energy of Endo-product= -0.028353 Hartree/Particle
Activation energy of Exo-product= 0.030427 Hartree/Particle
Activation energy of Cheletropic product= -0.005216 Hartree/Particle
The Activation energy of Exo-product is highest and positive, indicates that the reaction will take place spontaneously.
(Activation energies through a barrier should never be negative Tam10 (talk) 15:16, 27 October 2016 (BST))
(This diagram doesn't correspond to any of the data you've shown Tam10 (talk) 15:16, 27 October 2016 (BST))
Conclusions
In conclusion the computational experiment has successfully simulated two of the pericyclic reactions by calculations of the cyclic transition state structures using TS(Berny) methods. Two different levels of theories were used: B3LYP/6-31G(d) and simi-empirical/PM6. One of the advantages of computational simulation is that it simulates an IR spectrum for the transition state of an reaction which would be impossible experimentally.
Reference
- Fleming, Ian (1978). Frontier Orbitals and Organic Chemical Reactions. London: Wiley. pp. 29–109. ISBN 0-471-01819-8
- Bondi, A. (1964). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), pp.441-451.doi:http://pubs.acs.org/doi/abs/10.1021/j100785a00
- http://www.meta-synthesis.com/webbook/49_pericyclic/pericyclic.html