Rep:Mod:TK2016
Analysis of NH3 molecule
Molecule name:Ammonia
Calculation method:RB3LYP
Basis set:6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -56.557769 au
Point group:C3V
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986267D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
NH3 |
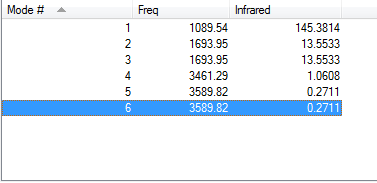
You expect 6 vibrational modes, calculated by the 3N-6 rule.
Modes 2&3 and modes 5&6 are degenerate.
The first three modes are bends and the last three are stretches.
The fourth mode is highly symmetric, with the frequency of ~3460 wavenumbers.
The first mode opens up and closes like an umbrella.
We expect 4 bands in the IR spectrum. But we can only see 2 of them and 1 of them is almost non-visible. This is probably due to the change in the dipole moment, which is not significant enough in other possible modes for it to be shown on the IR spectrum.
The charge we obtained by calculations is -1.125 for nitrogen and 0.375 for all 3 hydrogens. I did expect the nitrogen to have a negative charge, since it is more electronegative than the hydrogens and the hydrogens to have positive charge.
Analysis of N2 molecule
Molecule name:Nitrogen
Calculation method:RB3LYP
Basis set:6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -109.524129 au
Point group:D*H
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401127D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
N2 |
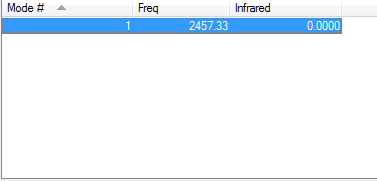
1 vibration was found for the nitrogen molecule at ~2460 wavenumbers. Since it is a homopolar dinuclear molecule, it is IR inactive, as the stretch does not result in a change in dipole moment.
Analysis of H2 molecule
Molecule name:Hydrogen
Calculation method:RB3LYP
Basis set:6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -1.178540 au
Point group:D*H
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
H2 |
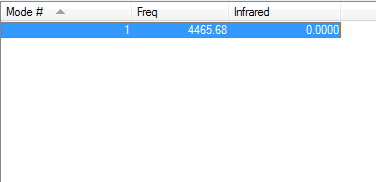
1 vibration was found for the hydrogen molecule at ~4450 wavenumbers. Since it is a homopolar dinuclear molecule, it is IR inactive, as the stretch does not result in a change in dipole moment.
Reaction energies for the production of Ammonia
E(NH3)=-56.55776873 au
2*E(NH3)=-113.11553746 au
E(N2)=-109.52412868 au
E(H2)=-1.17853936 au
3*E(H2)=-3.53561808 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.557907 au
ΔE=-146.47848285 kJ/mol=-146.48 kJ/mol
As we can see from the value above, the energy of of the product is lower than the energy of the reactants, therefore it is most likely to be thermodynamically favoured, but to say for sure, the enthropic contribution has to be checked as well.
We compared the value obtained to the literature value and obtained a literature value of -46.19 kJ/mol[1]. This value is significantly lower than the one Gaussview calculated. This due to the theoretical nature of the program, it makes a lot of approximations and does not take into account everything that occurs when preforming the reaction.
Analysis of SiH4 molecule
Molecule name:Silane
Calculation method:RB3LYP
Basis set:6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -291.888028
Point group:TD
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-2.453047D-14
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.4849 -DE/DX = 0.0 !
! R2 R(1,3) 1.4849 -DE/DX = 0.0 !
! R3 R(1,4) 1.4849 -DE/DX = 0.0 !
! R4 R(1,5) 1.4849 -DE/DX = 0.0 !
! A1 A(2,1,3) 109.4712 -DE/DX = 0.0 !
! A2 A(2,1,4) 109.4712 -DE/DX = 0.0 !
! A3 A(2,1,5) 109.4712 -DE/DX = 0.0 !
! A4 A(3,1,4) 109.4712 -DE/DX = 0.0 !
! A5 A(3,1,5) 109.4712 -DE/DX = 0.0 !
! A6 A(4,1,5) 109.4712 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -120.0 -DE/DX = 0.0 !
! D2 D(2,1,5,3) 120.0 -DE/DX = 0.0 !
! D3 D(2,1,5,4) -120.0 -DE/DX = 0.0 !
! D4 D(3,1,5,4) 120.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
SiH4 |
Vibrational analysis
You expect 9 vibrational modes, calculated by the 3N-6 rule.
The first three modes at 919.21 wavenumbers are degenerate, so are the three modes at 978.78 and 2254.9.
The first three modes are bends and the last three are bends.
The first mode is highly symmetric.
We can expect to see 2 bands in the IR, since 3 modes are IR inactive due to no change in the dipole moment, they are the highly symmetric modes. We get left with 6 modes then, of which 3 and 3 are degenerate. Therefore we can see 2 IR bands.
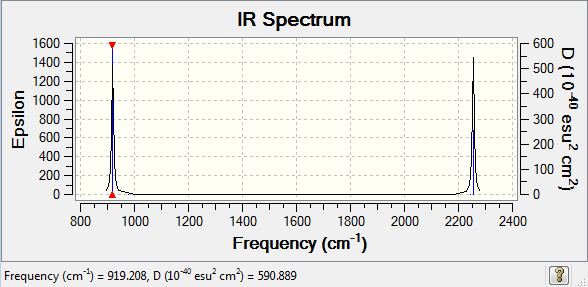
The first animation is the bend at 919.21 wavenumbers and the second animation is the stretch at 2254.9 wavenumbers. Both of these vibrational modes are IR active, due to the change in the dipole moment.
Charge analysis
The charge we obtained by calculations is 0.629 for Silicon and -0.157 for all 3 Hydrogens. I did expect the Silicon to have a positive charge, since it is less electronegative than the hydrogens and the hydrogens to have negative charge.
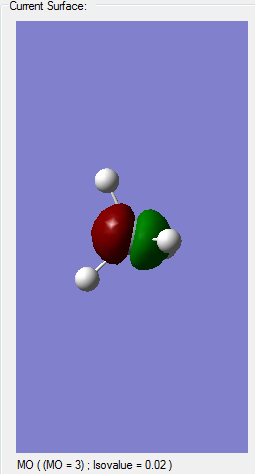
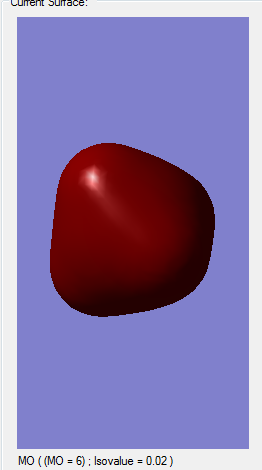
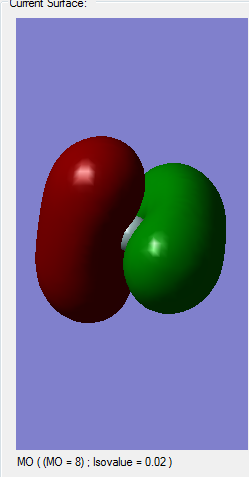
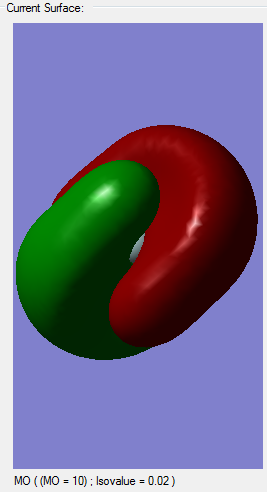
Molecular orbital analysis
Comparison of Silane's MOs to Methane's
Since I was concerned about whether the MOs were correct in the Silane calculation, I preformed another calculation of the molecule methane. Since carbon and Silicon are members of the same group, only one period apart I predicted that they are quite similar, bonding-wise. Methane is a tetrahedral molecule, with all the bonds equal due to the sp3 hybridization, where 2 electrons in the 2s orbital mix with 2 electrons from the 2p orbital and form 4 sp3 hybridized orbitals. I therefore predicted that the same would happen with Silane, with 2 electrons from 3s and two from 3p combining to form 4 sp3 orbitals. So by checking the calculation of methane, I should know whether this is true or not. In the sense of molecular orbitals, the results very similar for Silane and Methane, with some differences in energies and, of course, the extra electrons in Silane. I also measured the bond lengths for all Si-H bonds in Silane and all C-H bonds in Methane. Therefore all the bonds are identical and there is hybridization in both cases. I predict my assumption before, when describing molecular orbitals, was correct.
References
- ↑ http://www.chem.ox.ac.uk/vrchemistry/energy/table.htm ; 24.2.2017 at 16:50