Rep:Mod:THaywood/module one
Module 1
Hydrogenation of Cyclopentadiene Dimer
At room temperature clopentadiene can easily dimerise. There are two possible distereoisomers that could be formed; the endo and the exo form sown bellow.
 |
 |
It turns out that the endo isomer is predominantly formed. However the minimisation of the molecules shows that the exo isomer is thermodynamically more stable.
1) Exo Dimer Total Energy: 31.8783 kcal/mol
2) Endo Dimer Total Energy: 34.0083 kcal/mol
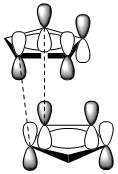
This suggests that the endo diastereoisomer is formed as the kinetic product of this dimerisation. This can be understood by looking at the frontier molecular orbitals of the two separate cyclopentadiene molecules as they approach each other during dimerisation.** Bellow is a diagram of the frontier orbital overlap that forms the new C-C bonds in the cyclopentadiene molecule. It shows how the molecules are help in place to favour the formation of the endo product. For the exo product to be formed these stabilising interactions must be totally absent, which is highly unfavourable.
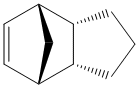
Partial hydrogenation of the cyclopentadiene gives one of the two following molecules.
 |
 |
3) Dihydro Derivative Stretch: 1.2321 Bend: 18.9145 Stretch-Bend: -0.7589 Torsion: 12.1503 Non-1,4 VDW: -1.5054 1,4 VDW: 5.7333 Dipole/Dipole: 0.1631 Total Energy: 35.9290 kcal/mol
4) Dihydro Derivative Stretch: 1.0980 Bend: 14.5041 Stretch-Bend: -0.5494 Torsion: 12.5004 Non-1,4 VDW: -1.0447 1,4 VDW: 4.5108 Dipole/Dipole: 0.1407 Total Energy: 31.1599 kcal/mol
Out of the two comfomers, isomer 4 is the least strained of the two so is thermodynamically more stable, this can be seen from the lower total energy. The main contribution for the difference in energy comes from the difference in the bending energy, the positionig of the double bond in 4 alows it to be moer flexible than 3 because the bond is in a 6 membered rign rather than a 5 membered ring. In bothe cases the double bonds are sp2 hybridised so have a desired bond angle of 120o. The angles in 4 are closer to this at 112o and 113o as opposed to 108o in 3 therefore experience less strain.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
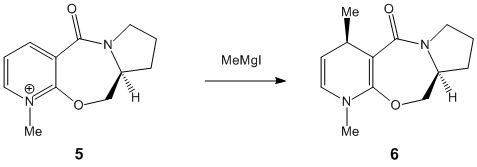
|
Molecule 5 is a derivative of proline, it reacts with MeMgI to alkylate the 4 position of the 5 membered ring. The methyl group faces up which can be seen from the product diagram. The explanation for the stereoselectivity can be understood by examening a 3D model of the reactant. It can be seen that the orientation of the carbonyl group with respect to the position of alkylation is slightly above the plane, the Mg coordinates with this oxygen meaning that the Me group always attacks on the top face of the ring. If the MeMgI is included in the calculation the program returns an error message. |
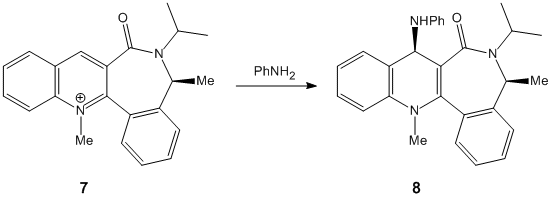
|
The next molecule is slightly different in that, this time the group adds on the opposite face to the carbonyl group; in this case the carbonyl group is now pointing down. This is because the reacting group is not able to coordinate to the lone pair on the carbonyl, therefore it simply attacks on the least hindered face, which is the face opposite to the side of the carbonyl. the steric hinderance is particularly strong due to lone pair repulsion. |
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
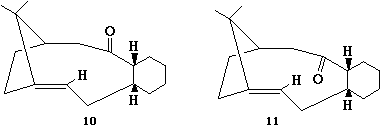
The two isomers were drawn and MM2 calculation carried out on them, the results show that the molecule 11 with the carbonyl pointing down has a lower energy so is more stable.
Molecule 10 Molecule 11 Stretch: 2.8238 Stretch: 2.5473 Bend: 16.4379 Bend: 10.6861 Stretch-Bend: 0.4565 Stretch-Bend: 0.3245 Torsion: 21.3913 Torsion: 19.5705 Non-1,4 VDW: -0.8783 Non-1,4 VDW: -1.1586 1,4 VDW: 14.0512 1,4 VDW: 12.5656 Dipole/Dipole: 0.1367 Dipole/Dipole: -0.1821 Total Energy: 54.4191 kcal/mol Total Energy: 44.3533 kcal/mol
The main contribution to the difference in the energies arrises from the difference in bending energies. This raised value in molecule 10 is due to the difference in the carbonyl bond angle. In both cases the carbonyl carbon is sp2 hybridised so prefers to be at 120o. The bond angle in 10 is 126o where as 11 has a bond angle of 120o explainging the difference in bending energy.
The explanation for these alkenes being particularly unreactive is due to the fact that if the double bond were to be hydrogenated then the molecule would be at a higher energy due to the strain of the ring that contains them. This phenomenon only occours in alkenes that are constrained with in a ring and give a negative value for the Olefin Strain energy. Alkenes which have these properties are labled hyperstable.
Modelling Using Semi-empirical Molecular Orbital Theory.
Regioselective Addition of Dichlorocarbene
Part 1
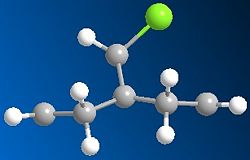
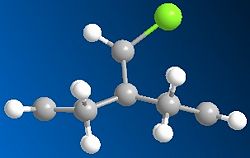
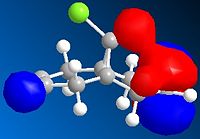
Running MOPAC/PM6 method on the dichlorocarbene molecule gives a approximate representation of the sturcture. This method, despite being aproximate still destinguishes between the two alkene bonds, as can be seen from the differing bond angles in the immage below.
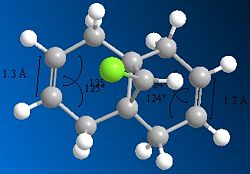
There is also a slight visual difference in the two optimised molecules, the one optimised using MM2 has a slight bend in the ring under the Cl where as the one run using MOPAC/PM6 is much flatter.
 |
 |
 |
 |
 |
 |
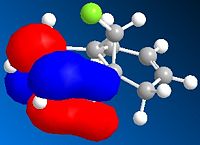 |
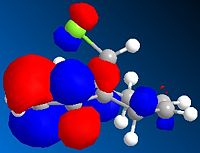
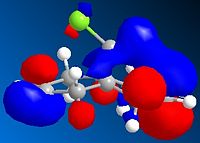
The diagrams above show various HOMO and LUMOs, it is clear that the HOMO of the double bond under the Cl are much larger than the HOMO on the of the double bond anti to the Cl, this shows that the bond under the Cl is therefore much more nucleophylic. As expected the LUMO on the double bond anti to Cl is much larger than the other double bond, this shows that the double bond anti to Cl is more electrophylic than the other.
Part 2
| Bond | Frequency | IR value |
|---|---|---|
| C=C (exo/anti) | 1737.09 | 4.2021 |
| C=C (endo) | 1757.36 | 3.9352 |
| C-Cl | 770.90 | 25.1197 |
The most obvious obsevation is that there is a difference between the vibrational frequencies of the two C=C bonds. This difference shows that the Cl bond has an effect on the anti peri planar C=C bond. The higher frequency of the endo bond suggests that it is stronger than the anti bond. This makes sence as the Cl-Cσ* orbital will overlap with the C=Cπ orbital drawing electron dencity from both bonds making them weaker than expected.
| Bond | Frequency | IR value |
|---|---|---|
| C=C | 1758.7 | 4.2971 |
| C-Cl | 776.51 | 20.3363 |
Hydrogenation has left the C=C (exo/anti) bond virtually unchanged. However the energy of the C-Cl bond has increased slightly. This is because the Cl-Cσ* orbital no longer interacts with the C=Cπ orbital, therefore has more electron dencity in the bond itself making it stronger so giving it a higher frequency.
Mini Project
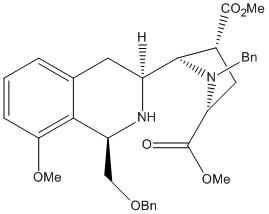
In the Synthesis of (-)-Quinocarcin via Aryne Annulation, there is a step where a 3.3:1 mixture of unstable diastereomeric dihydroisoquinolines are firmed. Treatment of this mixture with sodium cyanoborohydride resulted in a completely stereoselective reduction to produce an equivalent ratio of separable tetrahydroisoquinolines, the major diastereomer of which corresponds to the stereochemistry of the target alkaloid. It is these tetrahydroisoquinolines which are to be investigated.
 |
 |
Tetrahydroisoquinoline 19a
19a |
 |
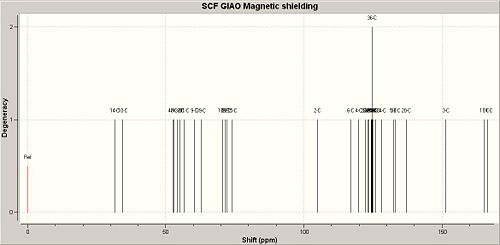 |
 |
| Literature Value | Calculated Value | Difference in ppm |
| 176.1 | 166.67 | 9.43 |
| 174.4 | 165.37 | 9.03 |
| 157.1 | 151.38 | 5.72 |
| 139.4 | 137.10 | 2.30 |
| 139.1 | 133.14 | 5.96 |
| 138.7 | 132.35 | 6.35 |
| 129.3 | 128.14 | 1.16 |
| 128.4 | 125.84 | 2.56 |
| 127.8 | 124.95 | 2.85 |
| 127.5 | 124.77 | 2.73 |
| 127.4 | 124.34 | 3.06 |
| 127.1 | 123.53 | 3.57 |
| 124.4 | 122.35 | 2.05 |
| 121.8 | 119.83 | 1.97 |
| 108.2 | 104.93 | 3.27 |
| 75.2 | 74.02 | 1.18 |
| 73.1 | 72.02 | 1.08 |
| 72.0 | 71.46 | 0.54 |
| 66.8 | 70.67 | -3.87 |
| 59.1 | 62.70 | -3.60 |
| 55.3 | 60.35 | -5.05 |
| 53.6 | 56.78 | -3.18 |
| 53.4 | 54.97 | -1.57 |
| 52.3 | 54.12 | -1.82 |
| 51.8 | 53.00 | -1.20 |
| 45.0 | 52.56 | -7.56 |
| 42.9 | 34.41 | 8.49 |
| 34.5 | 31.46 | 3.04 |
Tetrahydroisoquinoline 19b
19a |
 |
 |
 |
| Literature Value | Calculated Value | Difference in ppm |
| 176.2 | 172.96 | 3.24 |
| 174.3 | 172.86 | 1.44 |
| 156.6 | 151.24 | 5.36 |
| 139.2 | 137.03 | 2.17 |
| 138.2 | 136.63 | 1.57 |
| 136.9 | 134.27 | 2.63 |
| 129.8 | 124.68 | 5.12 |
| 128.5 | 124.56 | 3.94 |
| 128.3 | 124.09 | 4.21 |
| 127.8 | 123.53 | 4.27 |
| 127.6 | 123.38 | 4.22 |
| 127.4 | 123.28 | 4.12 |
| 123.8 | 122.42 | 1.38 |
| 121.6 | 120.99 | 0.61 |
| 107.7 | 104.32 | 3.38 |
| 72.8 | 74.12 | -1.32 |
| 71.5 | 72.37 | -0.87 |
| 69.4 | 71.74 | -2.34 |
| 66.8 | 60.98 | 5.82 |
| 59.2 | 60.53 | -1.33 |
| 55.4 | 54.87 | 0.53 |
| 52.3 | 54.38 | -2.08 |
| 51.8 | 52.68 | -0.88 |
| 47.4 | 51.69 | -4.29 |
| 42.7 | 50.35 | -7.65 |
| 34.1 | 37.30 | -3.20 |
| 32.4 | 35.10 | -2.70 |
Analysis
The majority of the chemical shifts from the calculated 13C NMR are fairly close to those quoted in the literature. Most of the values are within 5ppm of the literature values with just a few exceptions, these anomolies are likley to be due to the flexability of the molecule, this flexability allows variation in the environment of the carbons which in turn changes their chemical shift. The calulated NMR also lists more values than the literature, this is because Gausian does not take into account the affect of aromaticity and that some shifts are the same, in the literature NMR these peaks merge into a single broader peak.
References
1. JACS: Published on Web 11/26/2008: A Concise Total Synthesis of (-)-Quinocarcin via Aryne Annulation: Kevin M. Allan and Brian M. Stoltz: DiVision of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125
2. Supporting Information: A Concise Total Synthesis of (–)-Quinocarcin via Aryne Annulation: Kevin M. Allan and Brian M. Stoltz: The Arnold and Mabel Beckman Laboratories of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125, USA
