Rep:Mod:Skilganon2
The Hydrogenation of Cyclopentadiene Dimer
Endo versus Exo Cyclopentadiene
The Calculated Endo conformation of Cyclopentadiene(conformation 2):
Stretch: 1.2507 Bend: 20.8483 Stretch-Bend: -0.8357 Torsion: 9.5109 Non-1,4 VDW: -1.5449 1,4 VDW: 4.3206 Dipole/Dipole: 0.4477
Total Energy: 33.9975 kcal/mol
and the calculated Exo conformation of Cyclopentadiene(conformation 1):
Stretch: 1.2856 Bend: 20.5792 Stretch-Bend: -0.8380 Torsion: 7.6571 Non-1,4 VDW: -1.4171 1,4 VDW: 4.2322 Dipole/Dipole: 0.3776
Total Energy: 31.8766 kcal/mol
The largest contribution towards the difference in energy is the torsion angle. The exo product is more stable by virtue of torsional strain(lower degree of steric congestion), while the endo product is favoured by orbital overlap in the transition state. As a general rule, kinetic control takes place in the endo conformation, while the exo isomer(more thermodinamically stable) forms at a higher temperature.
However, the selectivity at room temperature is dictated by Baldwin's Rule, and in this case a favoured transition state geometry for effective orbital overlap is more desirable, and the endo isomer is the main product. At higher temperature, the thermodinamically more stable product is formed.
It should also be noted that Bredt's rule plays a role in determining the shape of the molecules. Bredt's rule is an empirical observation that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough.
Selectivity of the Hydrogenation of the Endo Dimer
The Calculated Hydrogenation for Dihydro Derivative 4(conformation 4):
Stretch: 1.0969 Bend: 14.5214 Stretch-Bend: -0.5493 Torsion: 12.4985 Non-1,4 VDW: -1.0679 1,4 VDW: 4.5119 Dipole/Dipole: 0.1406
Total Energy: 31.1520 kcal/mol
The Calculated Hydrogenation for Dihydro Derivative 3(conformation 3):
Stretch: 1.2756 Bend: 19.8651 Stretch-Bend: -0.8339 Torsion: 10.8082 Non-1,4 VDW: -1.2242 1,4 VDW: 5.6322 Dipole/Dipole: 0.1621
Total Energy: 35.6851 kcal/mol
From the calculation above it appears that the conformation 4 endo dimer is the more stable substituted dimer between the two. There is a large difference in energy between the two different substituted compounds. The bending energy contributes the largest to the difference(approximately 5.3kcal/mol), while the torsional is the next largest contribution to the difference. This could be explained by the shapes of both the conformer - by observing conformation 3 (pentadiene substituted compound) we can see an upwards twist in the furthest carbon atom. This suggests that the substituted conformer is highly strained and relieves its pressure by bending upwards, which explains its high torsional strain.
At room temperature, conformation 3 is more likely to exist, whereas at higher temperatures, the more stable conformer 4 takes place due to equilibrium.
Both the taxols seen below are different only in the position of the carbonyl group. The conformation seen below has its carbonyl group positioned directly anti syn to the neighbouring hydrogen, the calculated energy is seen directly below:
Taxol:The most stable conformation(10) |
Stretch: 2.6340 Bend: 13.9694 Stretch-Bend: 0.3504 Torsion: 20.9828 Non-1,4 VDW: -1.5695 1,4 VDW: 13.9405 Dipole/Dipole: -1.5838
Total Energy: 48.7238 kcal/mol
And the MMFF94 Minimisation shows a slightly higher value: Final Energy: 66.6127 kcal/mol
The conformation below has its carbonyl group in a gauche position to the Hydrogens. It would appear that the calculation suggests that this conformation is slightly less stable than the above conformation. The torsional strain in the above conformation is lower than the torsional strain of the below conformation, which is expected. The difference that contributes to the slight instability in the above conformation is the Bending Energy. A few calculations were run as seen below as we noticed that by constantly tweaking the conformation ever so slightly, one could obtain an arguably more stable conformation.
Taxol:The most stable conformation(10) |
Stretch: 3.1541 Bend: 19.0350 Stretch-Bend: 0.4464 Torsion: 23.4580 Non-1,4 VDW: 0.0933 1,4 VDW: 15.5402 Dipole/Dipole: -1.8340
Total Energy: 59.8929 kcal/mol
Stretch: 3.2568 Bend: 19.2504 Stretch-Bend: 0.3323 Torsion: 20.6342 Non-1,4 VDW: 0.7632 1,4 VDW: 15.3755 Dipole/Dipole: -1.8297
Total Energy: 57.7828 kcal/mol
MM2FF94: Final Energy: 81.2911 kcal/mol
Another conformation was tested to check the validity of the stability of the conformations. It appeared that this conformation yielded an unnaturally high instability contributed by the non-1,4 VDW forces. its Van Der Waals forces were increased rather significantly as well, along with the Bend energy:
Stretch: 6.6322 Bend: 85.5655 Stretch-Bend: -0.2209 Torsion: 20.7717 Non-1,4 VDW: 1.4946 1,4 VDW: 31.4737 Dipole/Dipole: -2.7389
Total Energy: 142.9778 kcal/mol
This conformation is almost similar to the most stable conformation above, which goes on to show that the conformers must be at an exact position for it to be stable. The difference in MM2FF94 is due to the different methods used in the calculations. However, that is of little concern as the energies are still comparable relative to one another.
A highly unusual feature about this system is that it violates the brett's rule, which states that bridges are unlikely to form adjacent to alkenes. However both isomers are relatively stable compounds... this could be explained by presence of hyperstable alkenes. Olefinic strain contributes to the stability of the overall alkene system, the strain is caused by the change in structure between the alkene group i.e twisting, decreasing the HOMO-LUMO gap significantly and thus increasing reactivity. Hyperalkenes serve as an opposite of the olefinic strain, in which the MO is so stable that reactivity does not occur. This is because of the shape of the aromatic, in which it is stretched tightly, therefore the carbon double bond alkene cannot undergo twisting - the isomer is therefore unreactive.
Modelling Using Semi-empirical Molecular Orbital Theory
The endo stereoselectivity in Diels Alder cycloadditions was attributed to "secondary orbital" interactions, which the Molecular Mechanics approach cannot handle. Therefore, the Semi-empirical MO theory could illustrate the electronic aspects of reactivity, showing how the molecular orbitals should be taken into account for a holistic method of calculation, and how electrons influence bonds and derived spectroscopic properties.
Regioselective Addition of Dichlorocarbene to a diene
The first molecule was minimised using molecular mechanics while the second was minimised using MOPAC. Overlay of both method of calculation.
1 C(9)-C(34) 0.1194 1 Cl(12)-Cl(37) 0.1074 1 C(1)-C(26) 0.0963 1 H(25)-H(50) 0.1180 1 C(5)-C(30) 0.0904 1 C(4)-C(29) 0.0411
From the detailed calculation above we can see that there is an anchor point carbon 4 and carbon 29 in which both atoms overlay each other well. The largest difference in overlay is seen in carbon 9-carbon 34 and the hydrogen atom 25 and 50. The large difference in the zone shows that the MO effect is more pronounced in these regions and the Molecular Mechanics method of calculation is no longer accurate enough to give us an overall representation.
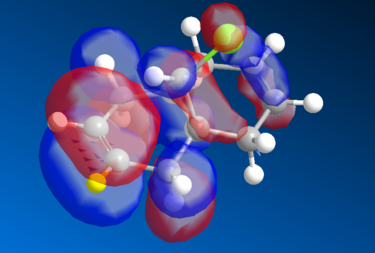
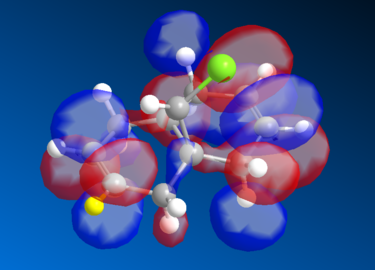
Molecular Orbital Analysis of the Diene
The Molecular Orbital of the Diene was generated using MOPAC method of calculation. The HOMO-1, LUMO, LUMO+1, and LUMO of the MO are seen as below.
 |
 |
 |
 |
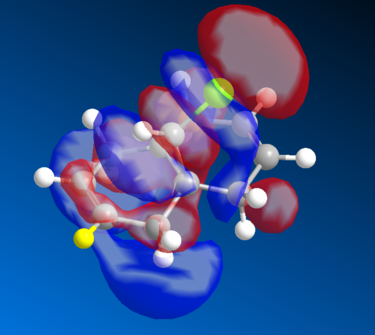 |
The HOMO-1 reflects the molecular symmetry well - overall we can see a relatively strong overall bonding between the MO's as compared to the HOMO, which makes sense as it is more stable overall than the HOMO. For the LUMO level, we start seeing an increase in antibonding MO particularly around the marked(yellow) hydrogen carbon group and its adjacent group, the MO symmetry still retains its shape, therefore the analysis is valid. There is a marked distinction between the LUMO and the LUMO+1 level of the diene - in the LUMO+1 there is a distinct increase in the antibonding orbitals seen opposite the (yellow) hydrogen bond. The orbital symmetry however is retained and is similar to the ones above. Finally, the LUMO+2 has extremely strong antibonding MO's, the red MO's around the aromatic ring is sandwiched between the 2 orbitals, creating a very strong antibonding energy and thus the energy is more unstable. Again, the symmetry is retained in the structure.
Molecular Electrostatic Potential
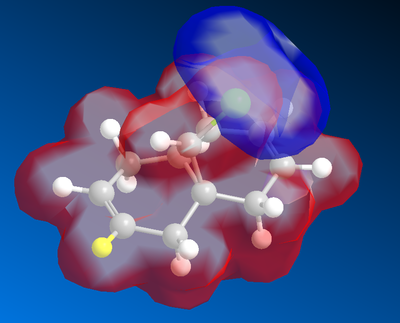
The Molecular electrostatic potential is seen as above, the potential was adjusted to show a clear contour between two electrostatic potential. The red surface represents the delocalised electron density. The chlorine atom, which is highly electronegative, is seen surrounded by a blue surface. As there is a distinct difference in the molecular electrostatic potential, the chlorine atom will constantly attract the electron density surrounding the aromatic to form an even contour.
From the diagram we can deduce that the molecular attack will occur from the endo position(opposite to the coloured hydrogen).
Molecular Vibration
The Molecular Vibration [[1]] was calculated via SCAN, and 69 different modes of vibrations were obtained, for convenience sake, we will just use frequencies with the highest intensities.
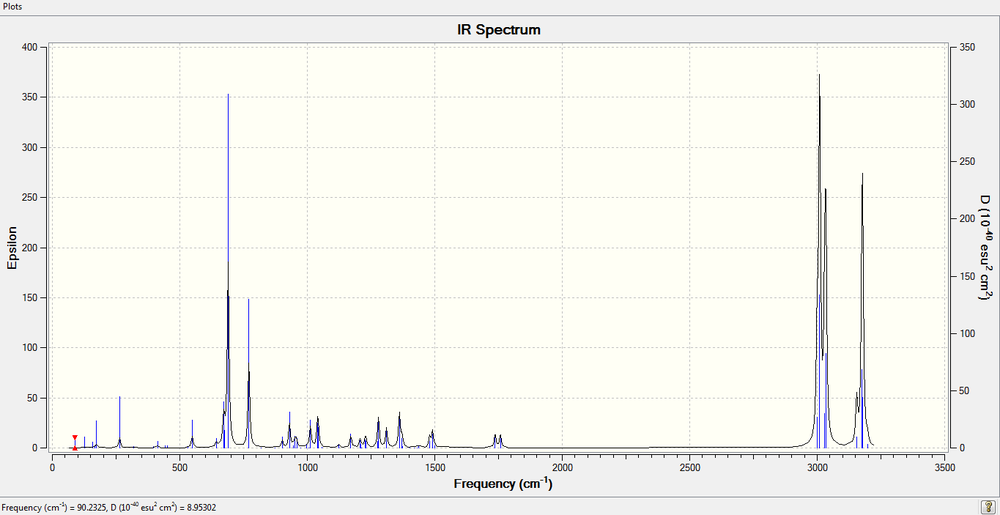
The above shows the overall spectrum of the diene vibration. As explained above, the peaks are relatively clean except for a few modes vibration with exceptionally high reading.
The 1737.04 vibration is what causes endo reaction to take place, whereas the 1757.34 vibration is what causes the exo reaction to take place.
Monosaccharide chemistry and the mechanism of gylcosidation
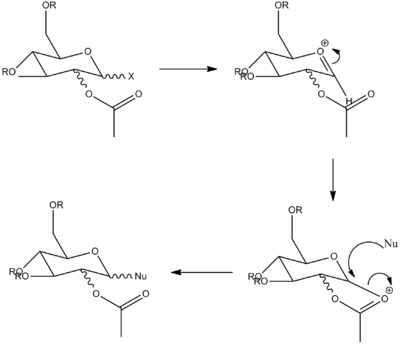
Gylcosidation reaction is highly specific. The stereochemistry of the C-OAc bond controls the stereochemical outcome of the C-Nu bond by nucleophilic attack. Therefore the objective of this analysis is to use MM2 and MOPAC to obtain a good calculation of the most stable stereoisomer.
Conformation A
MM2 Calculation
| Red Bond Stereoisomer Down | Red Bond Stereoisomer Up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conformation 1 | Conformation 2 | Conformation 3 | Conformation 4 | ||||||||||||
|
|
|
| ||||||||||||
Stretch: 2.8056
Bend: 8.4179 Stretch-Bend: 0.9401 Torsion: 4.1742 Non-1,4 VDW: 2.3914 1,4 VDW: 19.4867 Charge/Dipole: -32.5978 Dipole/Dipole: 8.4465 Total Energy: 14.0647 kcal/mol |
Stretch: 2.1932 Bend: 10.2062 Stretch-Bend: 0.8743 Torsion: 2.8637 Non-1,4 VDW: -1.7549 1,4 VDW: 19.3463 Charge/Dipole:-16.4796 Dipole/Dipole:5.6581 Total Energy:22.9073 kcal/mol |
Stretch: 2.5429 Bend: 12.5313 Stretch-Bend: 1.0197 Torsion: 3.1358 Non-1,4 VDW: 0.3824 1,4 VDW: 19.2395 Charge/Dipole: -23.6631 Dipole/Dipole: 7.2601 Total Energy:22.4486 kcal/mol |
Stretch: 2.2094 Bend: 10.5693 Stretch-Bend: 0.8279 Torsion: 2.0335 Non-1,4 VDW: -1.9149 1,4 VDW: 19.4674 Charge/Dipole: -17.5154 Dipole/Dipole: 5.0147 Total Energy:20.6920 kcal/mol | ||||||||||||
The conformer with the lowest energy is the more stable conformer and therefore most likely to occur - for red bond stereoisomer pointing downwards, conformation 1 is slightly more stable than conformation 2, and is therefore more likely to form. For red bond stereoisomer pointing upwards, conformation 4 is slightly more stable than conformation 3 and is therefore more likely to form.
Mopac Calculation
| Red Bond Stereoisomer Down | Red Bond Stereoisomer Up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conformation 1 | Conformation 2 | Conformation 3 | Conformation 4 | ||||||||||||
|
|
|
| ||||||||||||
| RMS Gradient = 0.09817 (< 0.10000) Heat of Formation = -69.56744 Kcal/Mol | RMS Gradient = 0.08243 (< 0.10000) Heat of Formation = -57.96477 Kcal/Mol | RMS Gradient = 0.08484 (< 0.10000) Heat of Formation = -77.33383 Kcal/Mol | RMS Gradient = 0.09983 (< 0.10000) Heat of Formation = -63.92546 Kcal/Mol | ||||||||||||
| Overlay of structure: Overall there is a greatest difference in the carbonyl oxygen overlap when comparing both structures. Structure | Overlay of structure: Overall there is a good overlap between both structures Structure | Overlay of structure | Overlay of structure | ||||||||||||
For the red bond with stereoisomer Down, conformation 2 is the more stable conformation however, instead of the above conformation 1. This shows the importance of including the MO's into consideration when calculating the overall stability. For Red bond stereoisomer facing up conformation 4 is the more stable conformation.
Comparison
By using the overlay operation of comparison, we noticed that both method of calculation for conformation 1 portrays a larger difference than the overlay calculation for conformation 2. The carbonyl oxygen overlap the most when comparing both structures for conformation 1. Energy is more stable in conformation 1 MM2 method of calculation, whereas MOPAC method of calculation reveals that conformation 2 is the more stable form isomer. The overlap reveals that the MM2 and MOPAC differs a lot more for conformation 1, therefore showing that conformation is not as stable as conformation 2 when the molecular orbitals are taken into account.
The bond length for both methods of calculations are the same. What differentiates the two overlaps are the bond angles, which could be seen from the mol file generated (a table is not generated because ultimately it will be confusing). Overall sugar A is more likely to be selected during a reaction because of neighbouring group participation.
Conformation B
Conformation 1 MM2 Calculation
Taxol:The most stable conformation(10) |
Stretch: 2.0146 Bend: 13.5355 Stretch-Bend: 0.7007 Torsion: 7.4524 Non-1,4 VDW: -2.4576 1,4 VDW: 17.7419 Charge/Dipole: -10.4939 Dipole/Dipole: -1.0290
Total Energy: 27.4646 kcal/mol
Conformation B only takes this shape as the aromatics are too tightly stable for it to be able to undergo further twisting.
Conformation 1 Mopac Calculation
Taxol:The most stable conformation(10) |
RMS Gradient = 0.09729 (< 0.10000) Heat of Formation = -77.34347 Kcal/Mol
Like the above, all the calculations of different methods of steroisomers reveal them to minimize to a same point, therefore only one conformation is valid.
Module 1 Mini Project: Simulation of Spectroscopic Data for a literature molecule
First Formatted Checkpoint File. [[2]] Second Calculated Taxol Derivative. [[3]]
Stretch: 4.9603 Bend: 18.6904 Stretch-Bend: 0.7241 Torsion: 24.2746 Non-1,4 VDW: 0.1010 1,4 VDW: 17.6239 Dipole/Dipole: -2.1570 Total Energy: 64.2173 kcal/mol
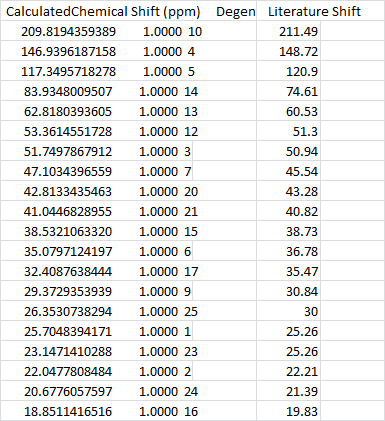
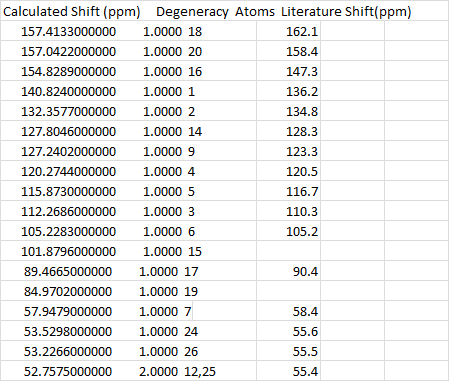
In this calculation, a change was made in the "solvent" module, that is it is replaced with benzene as a solvent due to literature. The Comparison in C 13 NMR is pretty good, as seen above. The main cause of the big difference in number 4 of the analysis is due to spin orbit coupling.
NMR Prediction and Analysis
Molecule of choice for study


The sketch on left and right shows both the E and Z conformation for the molecule. This is a minimized sketch via ChemBio3D and is therefore relatively accurate. The molecule on the left is called (Z)-2′,4′,6′-Trimethoxystyryl-4-methoxy-3-aminobenzyl sulfoxide and the molecule on the right is (E)-2′,4′,6′-Trimethoxystyryl-4-methoxy-3-aminobenzyl sulfoxide.
(Z)-2′,4′,6′-Trimethoxystyryl-4-methoxy-3-aminobenzyl sulfoxide is produced by first using solution of (Z)-2′,4′,6′-trimethoxystyryl-4-methoxy-3-nitrobenzyl sulfide (11, 0.5 g, 1.3 mmol) in 40 mL of glacial acetic acid was cooled to 5 °C on an ice bath. Hydrogenperoxide 30% w/v (0.20 mL, 2.0 mmol) was added and the mixture was stirred at 5 °C for 6 h. After completion of thereaction, 10% sodium hydrogen carbonate solution (25 mL)was added slowly and stirred for 10 min and extracted with ethyl acetate. The organic layer was washed with water (2 × 25 mL), dried over sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by column chromatography (hexane/ethyl acetate) to obtain the title compound.
The compound is further reduced using any reduction method to add a double bond O to the S atom.
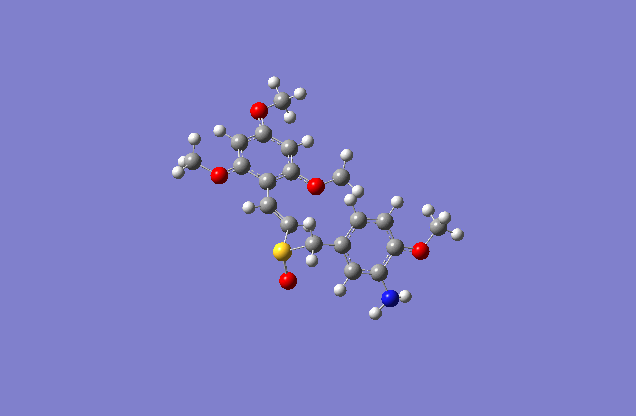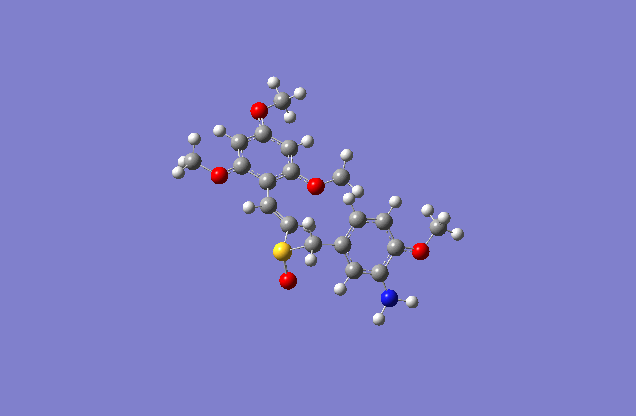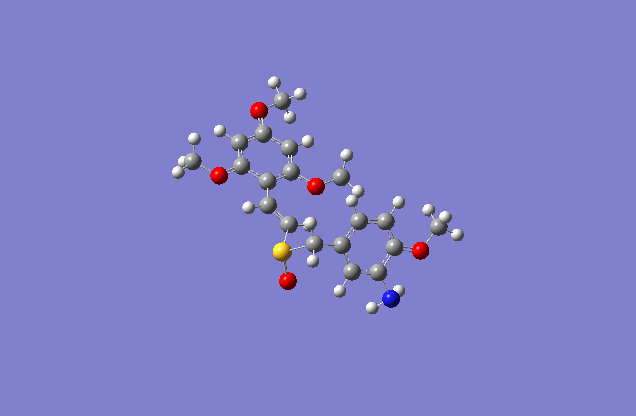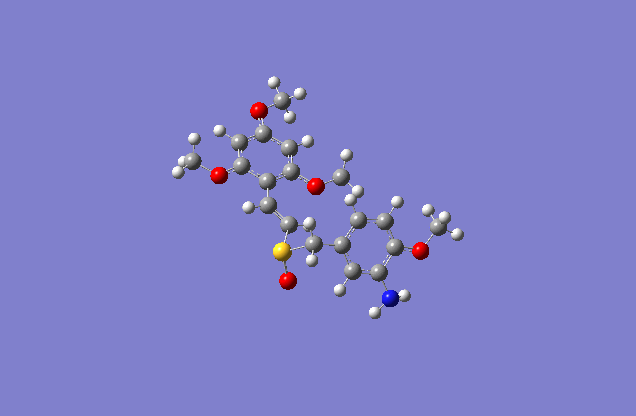
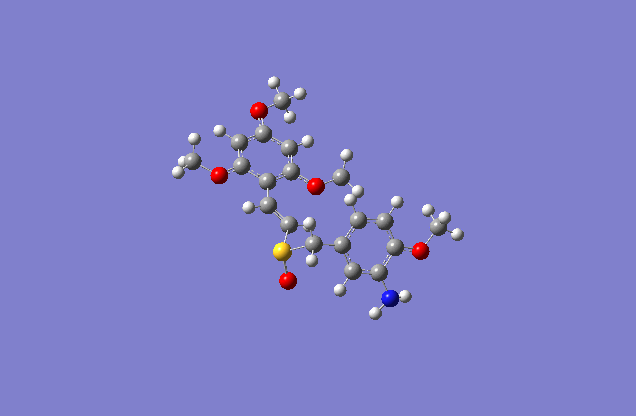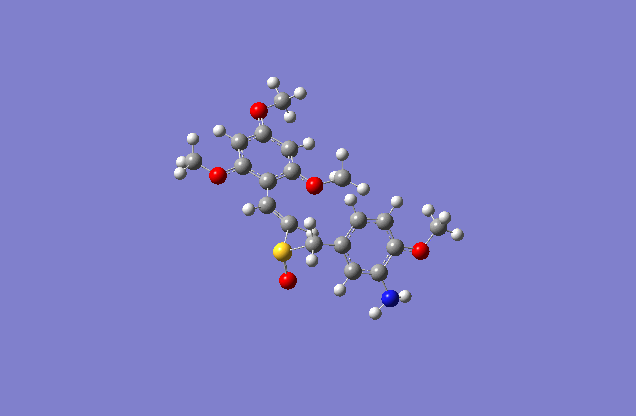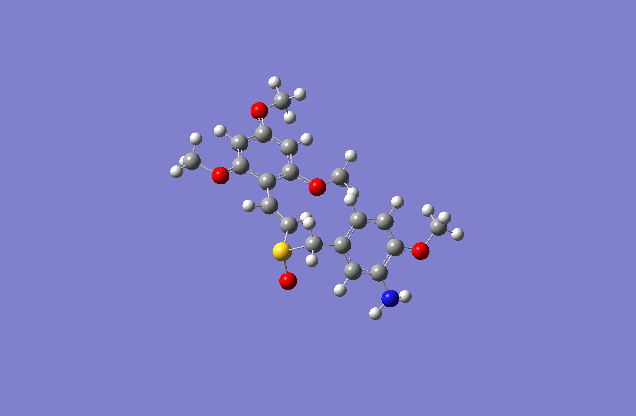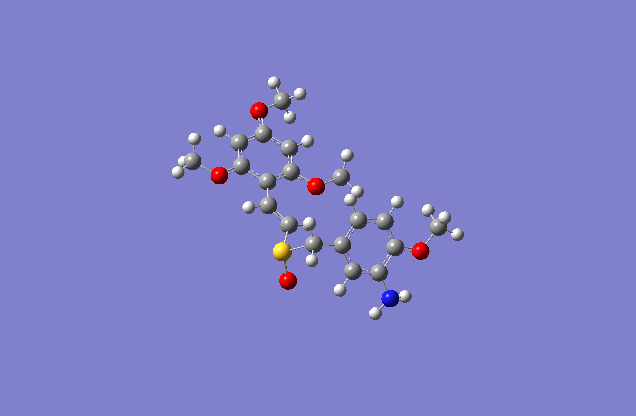
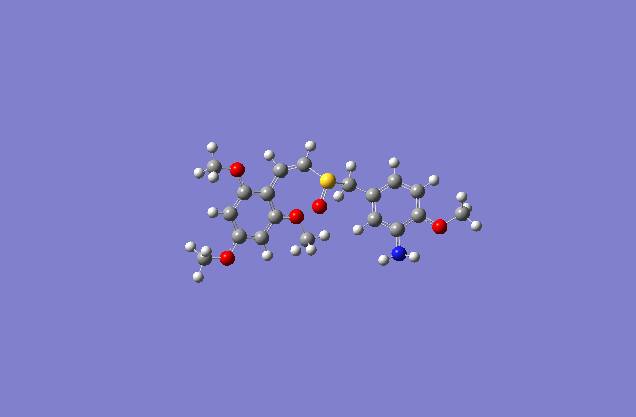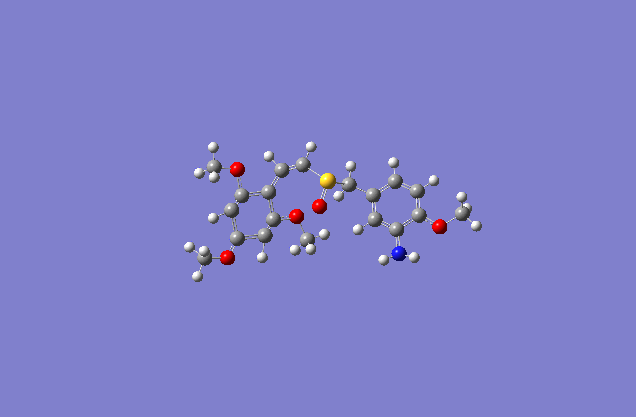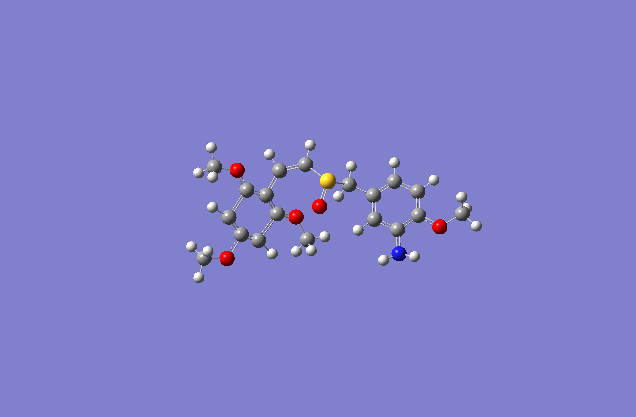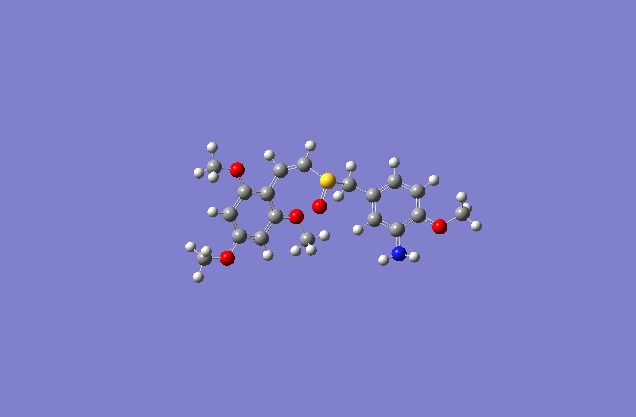
Taxol:The most stable conformation(10) |
The actual minimized isomer Z.
Taxol:The most stable conformation(10) |
The actual minimized isomer E.
NMR Analysis and Comparison
The NMR was generated from the optimised geometry of the isomer. For the Isomer with E conformation: [[4]] the J coupling was calculated as well! [[5]]
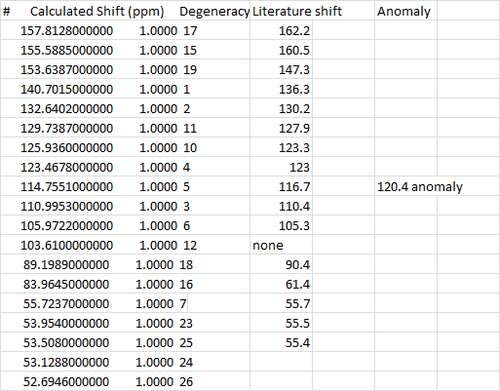
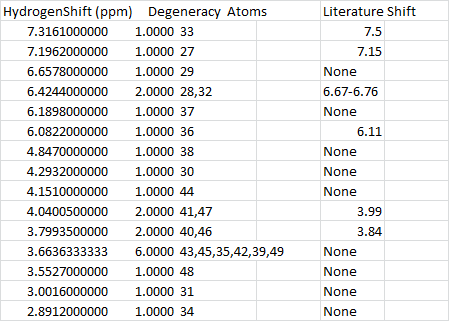
Overall, a relatively good fit was obtained for the C13 NMR, aside from a few gaps and differences. Bearing in mind that in the literature model, the compound may as well be contaminated, so certain shifts are likely to be missing, or extra shifts may be seen in this case. However in general, the ppm of literature and calculated values are within 5 ppm.
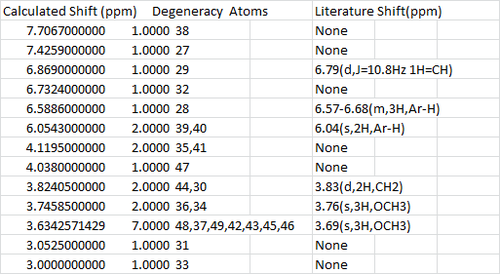
The H NMR however pose a larger problem as there are a lot more missing numbers from the literature value. However the values that fit the literature are good.
The NMR for the Z isomer was calculated: [[6]] and the J coupling [[7]], overall the fit is good.
Vibrational Analysis and Comparison
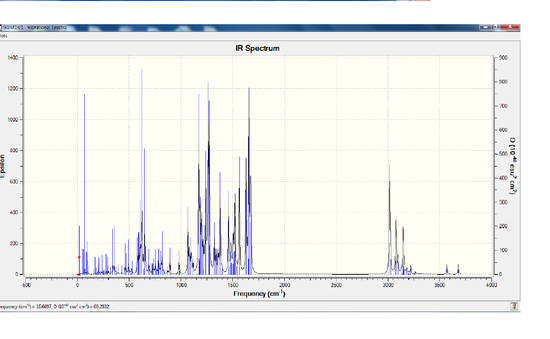
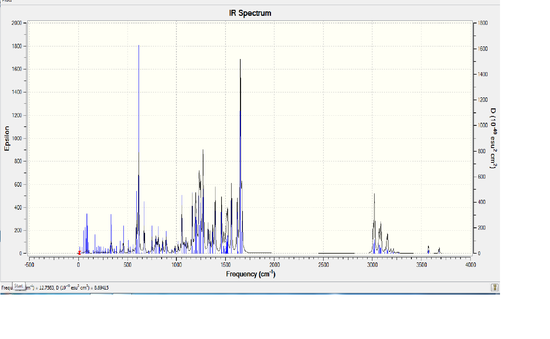
From the IR spectrum itself it looks as though isomer Z has the same spectrum as isomer E, however its insenties are a lot lower than that of isomer E. This means that the vibration for isomer Z is less intense than the vibration for isomer E. Below is a table which compares particular peaks of interest between the two molecules. D-space link Isomer E [[8]], Isomer Z [[9]] Isomer E has a Sum of electronic and thermal Energies= -1566.424099 HFT, 982931.122kJ/mol whereas Isomer Z has a Sum of electronic and thermal Energies= -1566.433798 HFT, -982937.21kJ/mol, there is only a very slight difference between the two energies.
Overall, no negative vibrations were obtained, therefore the calculated vibrations were not in their transition states.
Optical Rotation and Circular Dichroism
Optical Rotation
The Z optical Rotation was calculated [[10]]Optical Rotation Beta= -4.2889 au, [Alpha] ( 5890.0 A) = -439.64 deg and the E optical Rotation [[11]] Optical Rotation Beta= 4.5416 au, [Alpha] ( 5890.0 A) = 465.54 deg. Opposite, therefore it is valid as there shows a pair of isomers present.
Circular Dichroism
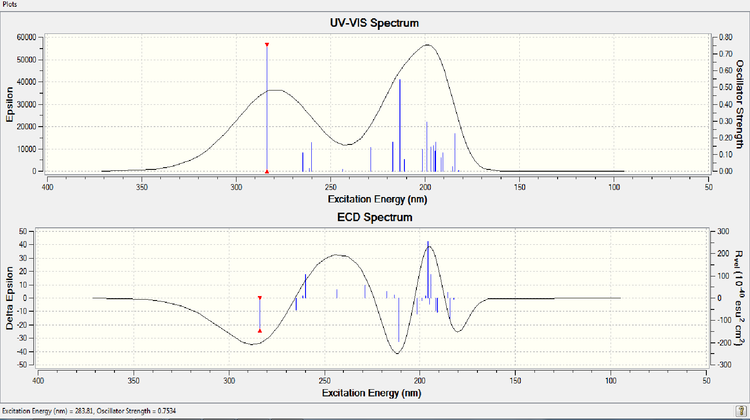
[[12]] Unfortunately, there are no references I could find to compare this to.
Reference
1.Strain energies of some bridgehead olefins as calculated with the MM2 force field Philip M. Warner, Stephen Peacock, Volume 3, Issue 3, pages 417-420. 2.Hydrothiolation of benzyl mercaptan to arylacetylene:application to the synthesis of (E) and (Z)-isomers of ON 01910·Na (Rigosertib®), a phase III clinical stage anti-cancer agent†,Venkat R. Pallela,‡a Muralidhar R. Mallireddigari,‡a Stephen C. Cosenza,b Balaiah Akula, a D. R. C. Venkata Subbaiah, b E. Premkumar Reddy b and M. V. Ramana Reddy* b