Rep:Mod:Shute
Intermolecular Interactions
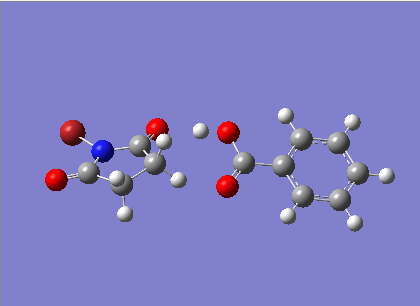
The prefered intermolecular interactions between a molecule of benzoic acid and NBS was investigated. Two likely possibilities were identified - a H bond interaction and a LB interaction between Br and O. This is assumed to be highly solvent dependent so chloroform model was used. This was selected under default in the solvents tab.
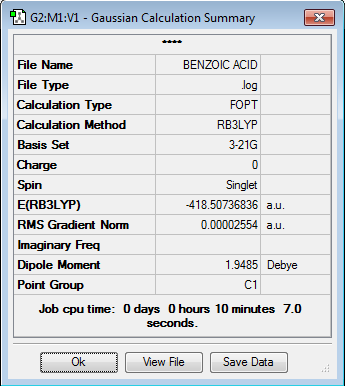
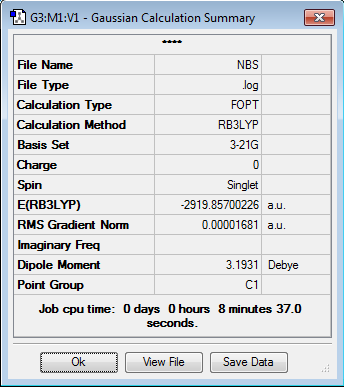
Initially, both molecules were optimised independantly:
It was considered surprising that the two CH2 groups in NBS were totally eclisped and that the structure was entirely flat.
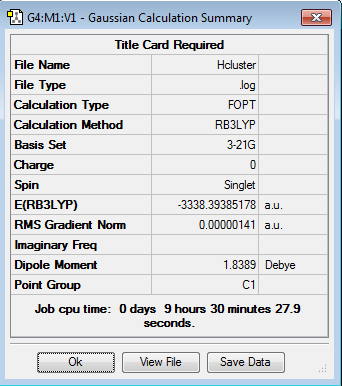
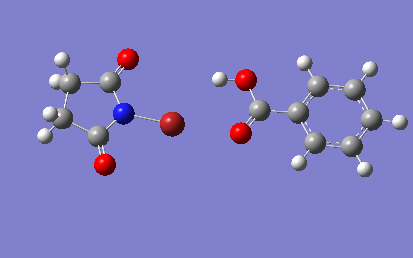
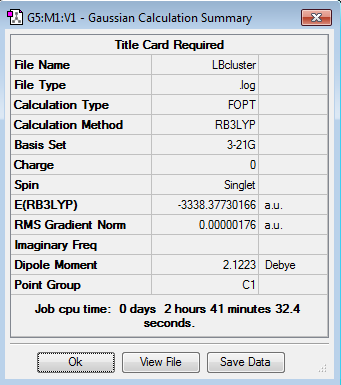
The two structures were added to a new molecule group together and optimised from appropriate starting arrangements giving the following results:
Interesting that NBS is now twisted slightly out of plane in the H bond case. What is average H bond length?
Notes on Method
shift+alt translates one molecule relative to another in a molecule group whilst alt rotates allowing them to be lined up approximately before optimisation. Viewing intermediate geoms in the log file gave an idea whether the molecule was converging correctly.
1) Job type Energy, 2) "pop=full" key word, 3) full NBO under the NBO tab and view chk file output.
REPEAT IN TOLUENE