Rep:Mod:SheridanClohessy
BH3
B3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000407 0.000450 YES RMS Force 0.000266 0.000300 YES Maximum Displacement 0.001617 0.001800 YES RMS Displacement 0.001058 0.001200 YES
BH3 frequency analysis log file
Low frequencies --- -0.0736 -0.0037 -0.0003 68.1470 68.1471 68.8261 Low frequencies --- 1164.5013 1214.1650 1214.1652
optimised BH3 molecule |
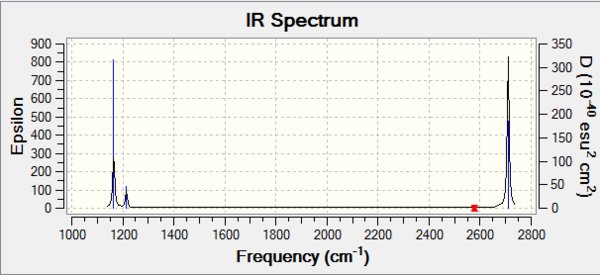
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1165 | 92 | A2" | yes | out-of-plane bend |
| 1214 | 14 | E' | yes | bend |
| 1214 | 14 | E' | yes | bend |
| 2577 | 0 | A1' | no | symmetric stretch |
| 2710 | 127 | E' | yes | asymmetric stretch |
| 2710 | 127 | E' | yes | asymmetric stretch |
- 6 vibrational modes are expected from the 3N-6 rule
- Modes 2 & 3 are degenerate so only give 1 peak; so are modes 5 & 6
- Mode 4 has no dipole moment, so is not IR active (so has no peak)
- Therefore only 3 peaks are seen on the spectrum
MO Diagram
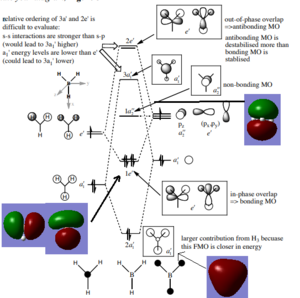
Are there any significant differences between the real and LCAO MOs?
Every single LCAO has the same basic structure as the real MOs. However, the qualitative LCAOs do not show extent of mixing between the fragment orbitals, and underestimate the size of the MOs.
Although not that accurate, the fact that they represent the shape of the real ones means they are a useful tool in exploring steroelectronic effects.
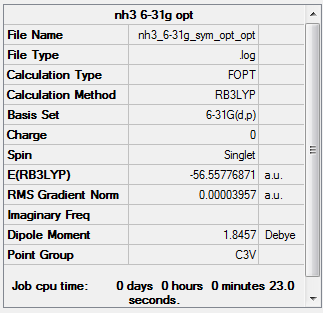
NH3
B3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000050 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.000220 0.001800 YES RMS Displacement 0.000096 0.001200 YES
Low frequencies --- -28.3665 -28.3664 -25.1016 -0.0012 0.0011 0.0032 Low frequencies --- 1088.2829 1693.7780 1693.7780
NH3 frequency analysis log file
optimised NH3 molecule |
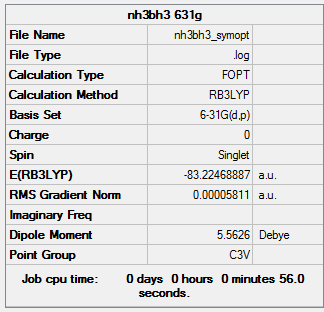
NH3BH3
B3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000138 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000765 0.001800 YES RMS Displacement 0.000181 0.001200 YES
Low frequencies --- -12.8312 -0.0300 -0.0051 0.0011 9.7169 9.7211 Low frequencies --- 262.7864 631.2150 638.0748
NH3BH3 frequency analysis log file
optimised NH3BH3 molecule |
E(NH3)= -56.26 a.u.
E(BH3)= -26.62 a.u.
E(NH3BH3)= -83.22 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE= -0.04 a.u.
ΔE= -100 kJ/mol
This represents the energy of formation of the B-N dative bond (so the bond entahlpy is +100 kJ/mol). It is quite weak compared to typical covalent bonds - for example the N-H bond energy in ammonia +450 kJ/mol. 1
Smf115 (talk) 19:25, 23 May 2018 (BST)Correct calculation and nice reference for the literature value referenced. However, the numbers being manipulated have been rounded too far and too early, full decimal places can be used for the calculation and the final value only has to adhere to the correct accuracy. The answer seems to have also been converted incorrectly from a.u. to kJ/mol.
BBr3
B3LYP/6-31G(d,p) level
The correct point group is D3H, I have spoken to a demonstrator who confirmed it is ok to use this optimisation.
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000026 0.001200 YES
Low frequencies --- -2.2682 -0.2047 -0.0002 -0.0001 0.0001 1.0519 Low frequencies --- 155.9355 155.9453 267.6878
optimised BBr3 molecule |
[N(CH3)4]+
B3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000124 0.000450 YES RMS Force 0.000048 0.000300 YES Maximum Displacement 0.000411 0.001800 YES RMS Displacement 0.000163 0.001200 YES
Low frequencies --- -0.0010 -0.0007 -0.0007 17.9443 17.9443 17.9443 Low frequencies --- 184.1249 289.8769 289.8769
[N(CH3)4]+ frequency analysis log file
optimised [N(CH3)4]+ molecule |
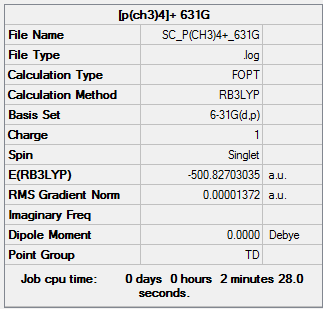
[P(CH3)4]+
B3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000065 0.000450 YES RMS Force 0.000015 0.000300 YES Maximum Displacement 0.000251 0.001800 YES RMS Displacement 0.000110 0.001200 YES
Low frequencies --- -0.0045 -0.0041 -0.0028 24.0481 24.0481 24.0482 Low frequencies --- 159.9671 194.7486 194.7486
[P(CH3)4]+ frequency analysis log file
optimised [P(CH3)4]+ molecule |
Analysis
| [N(CH3)4]+ | [P(CH3)4]+ | ||
|---|---|---|---|
| Atom | Charge | Atom | Charge |
| N | -0.295 | P | +1.667 |
| C | -0.483 | C | -1.060 |
| H | +0.269 | H | +0.298 |

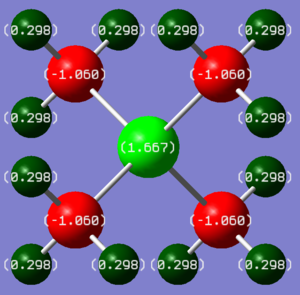
This charge distribution analysis has shown that the typical depiction of [NR4]+, with a positive charge on the nitrogen centre, is completely wrong. The positive charge is actually distributed over the hydrogen atoms, and the nitrogen atom has a slight negative charge. This makes sense for 2 reasons. Firstly, nitrogen is much more electronegative than hydrogen, so will attract electron density. Secondly, a comparison can be made with carbocations (carbon is only slightly less electronegative than nitrogen). Their positive charge is caused by electron deficiency - the carbon only has 6 valence electrons, and an empty p-orbital. As well as this, the positive charge is stabilised by sigma-conjugation with adjacent alkyl groups. In [N(CH3)4]+, the positive charge is caused by an extra -CH3+ group, and the valence orbitals are filled completely. This means that if the positive charge was centered on nitrogen, it would be a very unstable molecule.
The traditional picture is based on "formal charge", which assumes electrons from bonds are divided equally between atoms.
Conversely, [P(CH3)4]+ does have a large positive charge on the phosphorus atom. This is explained by the electronegativity difference: N > C > P/H. Its electropositive nature, and large size means it can more readily stabilise a positive charge. H cannot sustain the same level of positvie charge due to its small size - its nucleus is only a proton so the maximum it could have is +1.
Smf115 (talk) 23:14, 22 May 2018 (BST)Good explaination of the +1 charge location on the traditional description of [N(CH3)4]+ and on the real molecule. To improve the colour range to show the charge distribution should have been the same across the two molecules. Other features such as symmetry, or comparison of the similar H charges, could have been mentioned to develop the analysis further, although good discussion of electronegativities.
An interesting aspect of the MO analysis is the level of degeneracy - only 1/15 valence MOs is non degenerate. This is explained by the high symmetry of the compound (it has a Td point gorup_
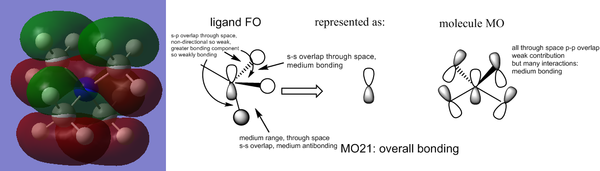

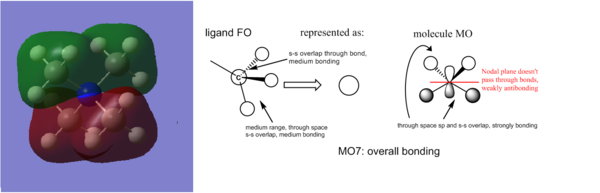
Smf115 (talk) 19:24, 23 May 2018 (BST)Nice comment about the degeneracy and clearly presented MO images. Great identification of the FOs and LCAOs with some additional comments on the interactions.
Smf115 (talk) 19:24, 23 May 2018 (BST)Overall a well presented wiki report and great project section.