Rep:Mod:SamH's page
Ammonia
Ammonia optimisation data
Calculation method: RB3LYP Basis set: 6-31G(d,p) Final energy: -56.55776873 au Point group: C3V
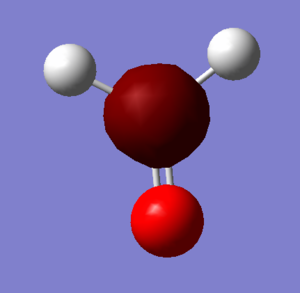
Image of Ammonia
Optimised Ammonia Molecule |
The bond length of the N-H bond in Ammonia is 1.01798 Angstroms. The H-N-H bond angle in Ammonia is 105.74118 degrees.
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
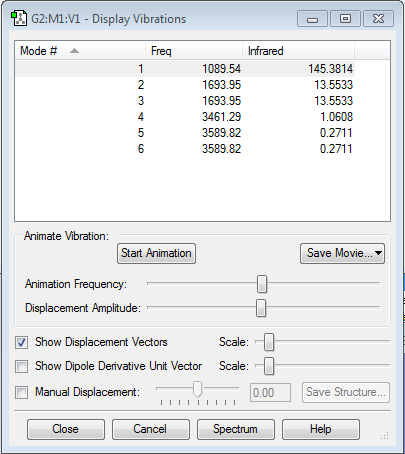
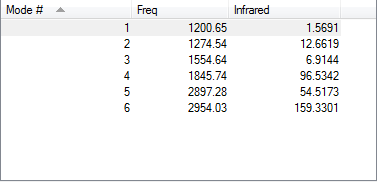
Vibrational information for Ammonia
From the 3N-6 rule we expect 6 as there is 4 different vibrational modes. Which the the number found. There are two sets of two which are degenerate, ie have the same energy.The modes 1, 2 and 3 are bending modes while modes 4, 5 and 6 are stretching modes. The modes 1 and 4 are highly symmetric. Mode 1 is known as the "Umbrella". Only two bands will show in the infrared spectrum as modes 1, 2 and 3 are too low in intensity to be registered and 4 and 5 are degenerate. So their band and the band for 6 show on the spectrum.
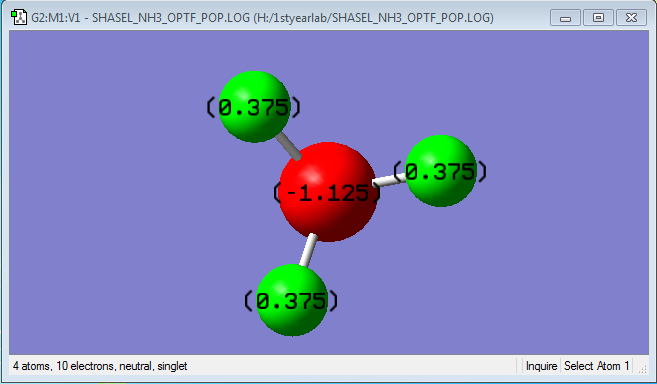
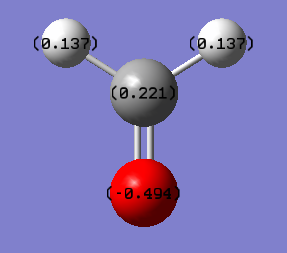
Charge information for Ammonia
The expected charge distribution was that the negative sign would be on the nitrogen, as found, as it is more electronegative than hydrogen. So they would have a positive number on their charge distribution.
Hydrogen
Hydrogen optimisation data
Calculation method: RB3LYP Basis set: 6-31G(d,p) Final energy: -1.17853936 au Point group: D∞h
Image of Hydrogen
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
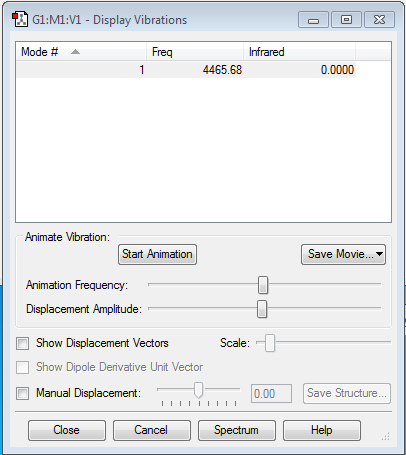
Vibrational information of Hydrogen
As the table shows there is one vibrational mode, we would predict this using the 3N-5 rule (as it is linear).
Charge information of Hydrogen
Nitrogen
Nitrogen optimization data
Calculation method: RB3LYP Basis set: 6-31G(d,p) Final energy: -109.52359111 au Point group: D∞h
Image of Nitrogen
The bond length of the N-N bond is 1.09200 Angstroms. The N-N bond angle is 180 degrees as the molecule is a linear diatomic.
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
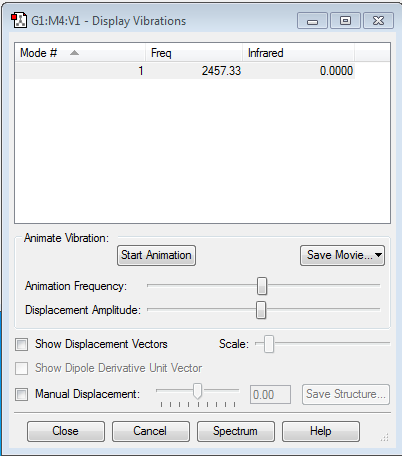
Vibrational information for Nitrogen
As the table shows there is one vibrational mode, we would predict this using the 3N-5 rule (as it is linear). The same result was found for diatomic hydrogen.
Charge information for Nitrogen

Haber-Bosch calculation
We can determine the energy for the reaction of N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 au 2*E(NH3)= -113.11553746 au E(N2)= -109.52359111 au E(H2)= -1.17853936 au 3*E(H2)= -3.53561808 au ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05632827 au or -147.889872885 Kj/Mol
The ammonia looks to be more stable as the energy change from the reactants to itself is negative, it is of a position of lower energy, hence it is more stable.
Methanal
Methanal optimization data
Calculation method: RB3LYP Basis set: 6-31G(d,p) Final energy: -114.50319933 au Point group: C2v
Image of Methanal
Optimised Methanal Molecule |
The bond length of C-H is 1.11056 angstroms for both of those bonds. The bond length of C=O is 1.20676 angstroms, which is longer than the C-H bond, even though double bonds are shorter than their equivalent single bonds, the hydrogens 1s orbital is very small which allows the bond to be shorter than the C=O bond. The O-N-H bond angle is 122.38589 degrees which is very close to the 120 degrees of a perfect trigonal planer molecule, the reason for it being slightly larger is that oxygen is relatively large so repels the hydrogen more than the hydrogen's repel each other. H-C-H has a bond angle of 115.21872 angstroms.
Item Value Threshold Converged?
Maximum Force 0.000197 0.000450 YES
RMS Force 0.000085 0.000300 YES
Maximum Displacement 0.000270 0.001800 YES
RMS Displacement 0.000149 0.001200 YES
Vibrational information of Methanal
From the table you can see that there are six different vibrational modes for methanal. The first frequency is due to H-C-H out-of-plane wagging but this is of very low intensity so may not show i=on the spectrum. The second frequency is due to H-C-H in-plane rocking and is of higher intensity than the first vibrational mode. H-C-H in-plane scissoring is responsible for the third vibrational frequency and is of low intensity. The fourth vibrational mode if caused by C-O stretching and is of very high intensity and will be easily observed on the spectrum. C-H asymmetric stretching is the cause of the fifth vibration which is intense and should be observed. The last vibrational mode that caused frequency six is C-H symmetric stretching and has the highest intensity of all of the vibrational modes so will definitely be observed.
Charge information of Methanal
The charge distribution shown is to be expected as oxygen is the most electronegative element of the molecule so should have the most electron density on it, hence the negative sign. Carbon and hydrogen have relatively similar electronegativities so are expected to have close positive values.
Analysis of selected MO's
This is the lowest energy molecular orbital of methanal, it looks to be completely formed from the 1s AO from oxygen as the MO is covering the oxygen atom with no electron density anywhere else. It is the lowest energy bonding MO as the electrons in the orbital are held tightly by the nucleus as the effective nuclear charge is strong. This MO will have a very very small effect on bonding as it is the lowest energy MO.
This is the second lowest energy MO and is the anti-bonding orbital of the MO above. The majority contribution of this MO is from the hydrogen as this MO is surrounding the hydrogen. This has no contribution to bonding of the molecule, as with the above orbital, because this orbital cancels out the bonding effect of the orbital above. As filling anti-bonding orbitals breaks the bond.
This is the third lowest energy MO, it is a bonding MO. The contributions of this seem to come from every atom in the molecule but the oxygen atom seems to contribute the most, this would be because it is the most electronegative atom in the molecule. It is an occupied orbital, as are the ones above. This also has no contributing to bonding as there is a corresponding anti-bonding orbital which is also filled which cancels out the bonding effect of this orbital.
This is the highest energy occupied molecular orbital (HOMO) which is an anti-bonding MO and wont contributes to the bonding of the molecule as its the anti-bonding orbital. The main contributors to this orbital are the two hydrogens mainly and the oxygen.
This is the lowest energy unoccupied molecular orbital (LUMO) which definitely does not contribute to bonding as the orbital is unoccupied. All the atoms in the molecule contribute to this MO being formed