Rep:Mod:SXC1322a
Experiment 1C: Advanced molecular modelling and assigning the absolute configuration of an epoxide
Conformational analysis using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
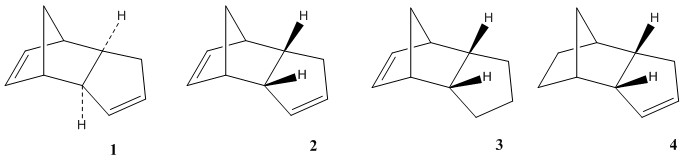
During the dimerization of the cyclopentadiene, both of dimer 1 and 2 can form; however, in fact, only dimer 2 forms. The dihydro derivatives 3 and 4 are initially produced by the hydrogenation of dimer 1 but only the tetrahydro one exists after the prolonged hydrogenation. By calculating the energies of the four species 1-4, we can infer that which specie is more stable.
The molecule geometries were optimised by using MMFF94s force field in Avogadro, with the structures of the species constructed in Chemdraw Pro. Therefore the energies of them can also be calculated in Avogadro.
Dimer 1
Dimer_1 |
Dimer 2
Dimer_2 |
Dimer 3
Dimer_3 |
Dimer 4
Dimer_4 |
| Dimer 1 (kcal/mol) | Dimer 2 (kcal/mol) | Dimer 3 (kcal/mol) | Dimer 4 (kcal/mol) | |
|---|---|---|---|---|
| Total Bond Stretching Energy | 3.54276 | 3.46589 | 3.31146 | 2.81369 |
| Total Angle Bending Energy | 30.76922 | 33.18499 | 31.98610 | 24.99990 |
| Total Stretch-bending Energy | -2.04133 | -2.08141 | -2.10578 | -1.63866 |
| Total Torsional Energy | -2.73368 | -2.96007 | -1.51657 | -0.29312 |
| Total Out-Of-Plane Bending Energy | 0.01424 | 0.02217 | 0.01286 | 0.00096 |
| Total Van Der Waals Energy | 12.80711 | 12.39890 | 13.63837 | 10.57677 |
| Total Electrostatic Energy | 13.01384 | 14.19724 | 5.11943 | 5.14812 |
| Total Energy | 55.37335 | 58.19416 | 50.44587 | 41.60766 |
Table.1 Result energy of dimer 1, 2, 3 and 4
From the simulation, Angle bending energy contributes the most among the total energies of these four species. Dimer 1 has lower energy than dimer 2, indicating the cyclodimerisation is under kinetic control; and similarly specie 4 is more thermodynamically stable due to the lower energy.
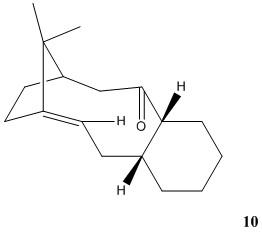
Taxol is a significant drug in treating ovarian cancers. Isomer 9 or 10, is an important intermediate in the synthesis of this drug, either with the carbonyl group pointing up or down, existing atropisomerism. By calculating the energies of the two isomers in the same way described above, the relative stability of them can be found out.
Isommer 9
Dimer_1 |
Isomer 10
Dimer_2 |
| Isomer 9 (kcal/mol) | Isomer 10 (kcal/mol) | |
|---|---|---|
| Total Bond Stretching Energy | 7.61695 | 8.96178 |
| Total Angle Bending Energy | 28.31531 | 22.96519 |
| Total Stretch-bending Energy | -0.10252 | -0.15088 |
| Total Torsional Energy | 0.50813 | 4.27377 |
| Total Out-Of-Plane Bending Energy | 0.97717 | 1.62090 |
| Total Van Der Waals Energy | 32.98055 | 36.81104 |
| Total Electrostatic Energy | 0.30750 | 0.40923 |
| Total Energy | 70.60309 | 74.89102 |
Table 2. Result energy of isomer 9 and 10
We can infer from the results that isomer 9 is more stable than isomer 10, whereas Van Der Waals energy is the largest contributor, therefore resulting two different stereochemistry of carbonyl addition of these two isomers. The lower bending energy of isomer 10 is due to the smaller bond angle, 50.3o, comparing to 51.8o of isomer 9. Calculated by MMFF94s force field, the olefinic strain, corresponds to the double bond twisting, lowers the difference between the HOMO and the LUMO and therefore more reactive. However, “hyperstable” olefins locate at the bridgehead and are thermodynamically more stable therefore highly unreactive. So the alkene reacts slowly.
Reference for this part
1. W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891.DOI:10.1021/ja00398a003
Spectroscopic Simulation using Quantum Mechanics
By using the powerful HPC system, the 1H and 13C spectra of molecules 17 and 18 are simulated. Therefore the calculated chemical shifts can be compared to the literature values.
Here, molecule 17 is choosen for the simulation.
Molecule 17
Dimer_1 |
| Isomer 17 (kcal/mol) | |
|---|---|
| Total Bond Stretching Energy | 15.57460 |
| Total Angle Bending Energy | 32.40643 |
| Total Stretch-bending Energy | 0.01688 |
| Total Torsional Energy | 11.58311 |
| Total Out-Of-Plane Bending Energy | 1.23413 |
| Total Van Der Waals Energy | 51.21533 |
| Total Electrostatic Energy | -7.56874 |
| Total Energy | 104.46173 |
Table 3. Result energy of molecule 9 and 10
| Energy | |
|---|---|
| Zero-point correction | 0.467966 (Hartree/Particle) |
| Thermal correction to Energy | 0.489479 |
| Thermal correction to Enthalpy | 0.490423 |
| Thermal correction to Gibbs Free Energy | 0.420577 |
| Sum of electronic and zero-point Energies | -1651.412064 kcal/mol |
| Sum of electronic and thermal Energies | -1651.390551 kcal/mol |
| Sum of electronic and thermal Enthalpies | -1651.389607 kcal/mol |
| Sum of electronic and thermal Free Energies | -1651.459453 kcal/mol |
Table 4. Calculated values for energies
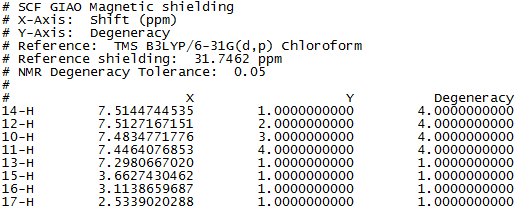
Figure 1 to 4 are the calculated 1H and 13C NMR data and spectra for molecule 17.
And figure 5 diplays the literature values of 1H NMR for molecule 17.
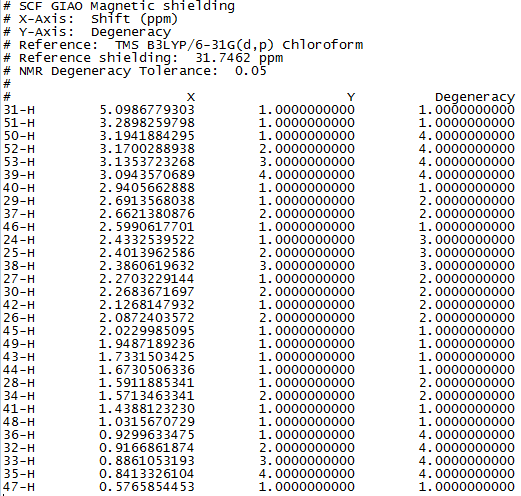

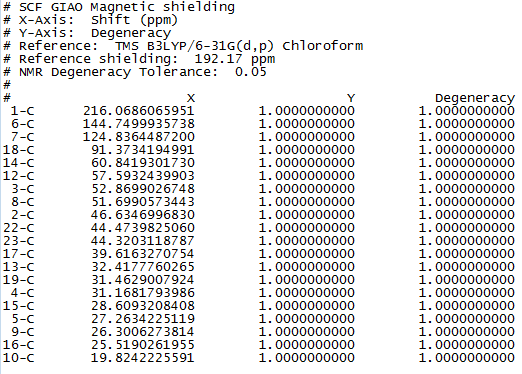

13C NMR (75 MHz, C6D6) ppm 211.49, 148.72, 120.90, 74.61, 60.53, 51.30, 50.94, 45.53, 43.28, 40.82, 38.73, 36.78, 35.47, 30.84.30.00, 25.56, 25.35, 22.21, 21.39
Literature 13C NMR data for molecule 17
It is noted that these simulated NMR spectra, with a reasonable range of error, are fairly similar to those in the literature.
Reference for this part
1. Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283.DOI:10.1021/ja00157a043
Analysis of the properties of the synthesised alkene epoxides
The two catalytic systems
The two catalysts, the shi asymmetric Fructose catalyst and the Jacobsen asymmetric catalyst, are used to catalyse asymmetric expodation of alkenes.
The Shi asymmetric Fructose catalyst
The Jacobsen asymmetric catalyst
These two molecules' structures are also simulated in Avogadro.
The Shi catalyst
Dimer_1 |
The Jacobsen asymmetric catalyst
Dimer_2 |
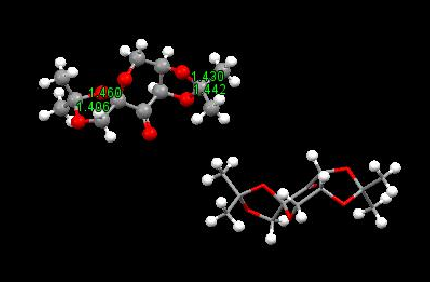
The crystal structures of the two catalysts above
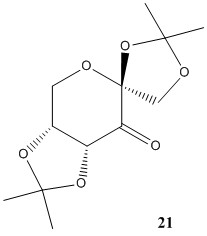
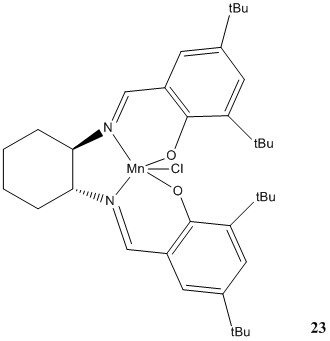
Molecule 21 and 23 are the pre-catalysts in the synthesis process of the Shi catalyst and the Jacobsen catalyst respectively.
Pre-catalyst 21
 Chloro-(N,N'-bis(3,5-di-t-butyl-salicylidene)cyclohexane-1,2-diamine)-manganese
Chloro-(N,N'-bis(3,5-di-t-butyl-salicylidene)cyclohexane-1,2-diamine)-manganese
The two catalysts were searched in the Cambridge crystal database(CCDC) by using the Conquest program. The C-O bond lengths in the molecules were then obtained using the Mercury program.
Figure 6. The C=O bond lengths of the anomeric centres
As shown in Figure. 6, the C-O bond lengths of the two anomeric centres can be investigated by using Mercury, which are 146, 140.6, 143 and 144.2 pm respectively, all different from the normal C=O bond length(143 pm). This shortening of bonds is due to the elongation of the bonds in the molecule. The lone pair on the oxygen donates its electron to the C-O bond results in the strengthening of that bond and the weakening of the neighbour C-O bond.
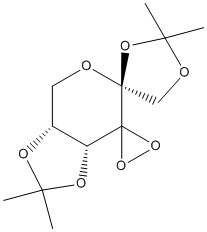
 1,2:4,5-Di-O-isopropylidene-b-D-erythro-hexo-2,3-diulo-2,6-pyranose
1,2:4,5-Di-O-isopropylidene-b-D-erythro-hexo-2,3-diulo-2,6-pyranose
Pre-catalyst 23
Epoxidation of alkene 1 and 2
The calculated NMR properties of the epoxidation products
The NMR of the epoxidation products are calculated by using the same method as the molecule 17 and 18. The optimised structures are as follows
1,2-dihydronaphthalene epoxidation product
Dimer_1 |
Styrene epoxidation product
Dimer_2 |
Figure 7 to 14 are the computed NMR spectra and data for epoxide 1 and 2.

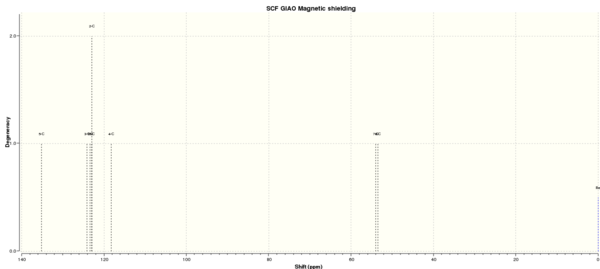
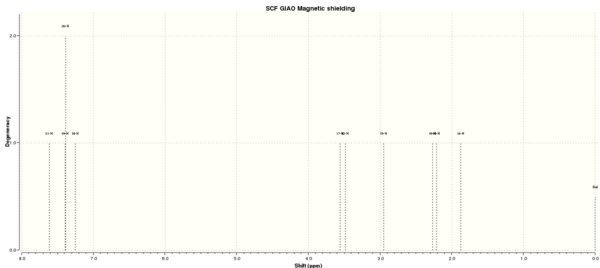
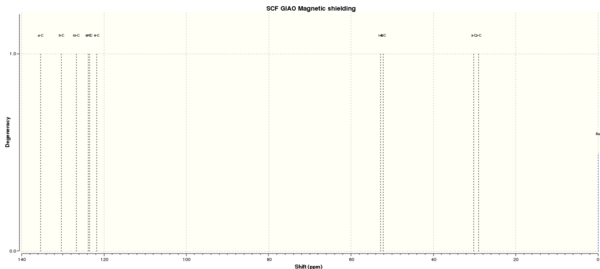
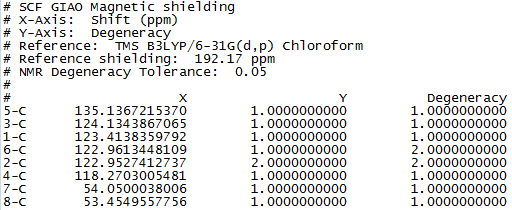
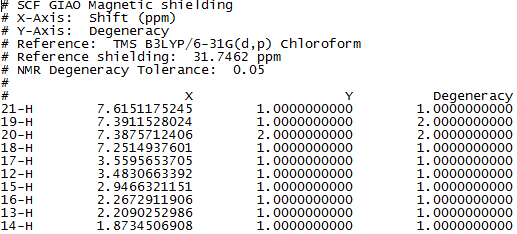
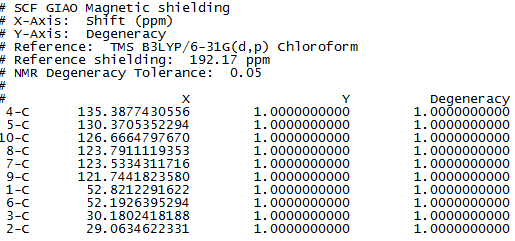
Assigning the absolute configuration of the epoxides
Using the checkpoint documents produced before, .gjf documents can be generated in Gaussview. By entering different codes in the NotePad++ program, the .gjf documents can be used for different calculations (e.g. ECD, VCD, optical rotary power) in the HPC system. The result spectra can be then obtained by viewing output .log documents in Gaussview and the values obtained simply in Notepad.
The reported literatures for optical rotations
1.The optical rotation at a specified wavelength of light (the optical rotatory power, or ORP)
OPR, the optical rotatory power, measures the optical rotation at a defined wavelength of light.
Table 5. Literature optical properties for epoxides
The calculated chiroptical properties of the product
Table 6. Calculated optical properties for epoxides
2. The electronic circular dichroism (ECD)
ECD, the electronic circular dichroism, is effective in recording with polarised light but not very helpful epoxides due to the lack of appropriate chromophore.


3. The vibrational circular dichroism (VCD)
VCD, the vibrational circular dichroism, is not available in our department but very helpful.
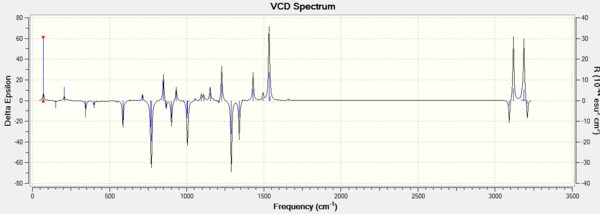
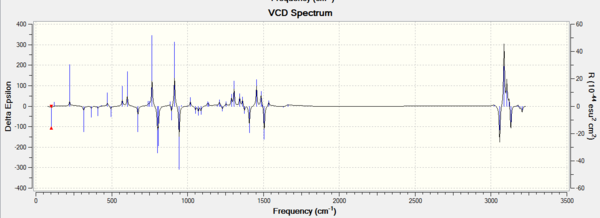
Using the (calculated) properties of transition state for the reaction (β-methyl styrene)
For checking the enantiommeric assignments, the relative free energy of the transition state obtained by computer simulation and the calculated optical rotations of the epoxide were compared.
The working on calculating the value of energy constant k are shown below.
| (R,R) | (S,S) | |
|---|---|---|
| Free energy, G of TS1 | -1343.02297 | -1343.017942 |
| Free energy, G of TS2 | -1343.019233 | -1343.015603 |
| Free energy, G of TS3 | -1343.029272 | -1343.023766 |
| Free energy, G of TS4 | -1343.032443 | -1343.024742 |
| Average Free Energy, G | -1343.02598 | -1343.020513 |
| Difference in Free Energy, G (in Hartree) | -0.00546625 | |
| Difference in Free Energy, G (KJ/mol) | -14351.6373 | |
| Value of k calculated | 326.917 |
Table 7. Calculation for constant k
From the calculations above in Table 7. , the free energy constant k = 326.917, indicating that the enantiomers with R,R stereochemistry is much more preferable over S,S enantiomers. And the value of the free energy is relatively large therefore the enantiomers are very stable.
Investigating the non-covalent interactions in the active-site of the reaction transition state
Hydrogen bonds,electrostatic attractions and dispersion-like close approach of pair of electrons, are all included in NCl, the non-covalent interactions. These analysis was done by using Gaussview.
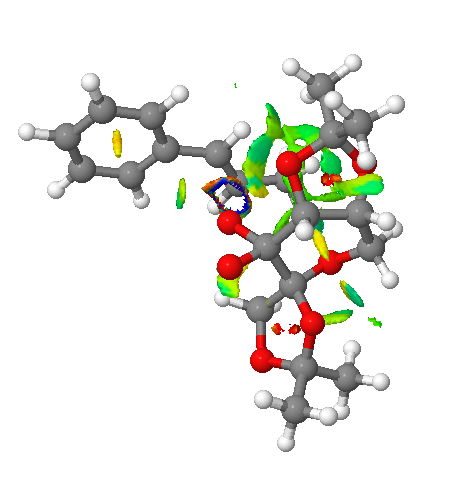
Figure 19. NCL analysis of R,R series for Shi expodation of β-methyl styrene
As Figure 19. displays above, attractive interactions are indicated by the different colours, therefore helpful for identifying regions of reacting system that reaction of substrate and catalyst may take place.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
QTAIM, the electronic topology, Focus on the weaker interactions defined by a NCl analysis and the electron density in the covalent area of species, is complementary to the NCl analysis.
Figure 20. QTAIM analysis of R,R series for Shi expodation of β-methyl styrene
Figure 20. above is the QTAIM analysis of R,R series for Shi expodation of β-methyl styrene. This was done in Avogadro program on a mac.
Suggesting new candidates for investigations
Through searching on Reaxys, a molecule with optical rotary power > -500 can be found out. Therefore (-)-lochnericine is chosen for discussion.
| Structure | 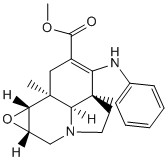
|
| Solvent | CHCl3 |
| Optical Rotation Power | -528o |
| Wavelength (nm) | 589 |
| Temperature | 15oC |
Table 8. Literature Optical Properties of (-)-lochnericine
Reference for this part
1. S. Pedragosa-Moreau , A. Archelas , R. Furstoss J. Org. Chem., 1993, 58 (20), pp 5533–5536 DOI:10.1021/jo00072a044
2. Journal of Molecular Catalysis B: Enzymatic, 2010 , vol. 67, # 3-4 p. 236 - 241 DOI:10.1016/j.molcatb.2010.08.012
3. Tetrahedron, 1996 , vol. 52, # 13 p. 4593 - 4606 DOI:10.1016/0040-4020(96)00135-4
4. Patent: US6348608 B1, 2002
5. Moza,B.K.; Trojanek,J. Collection of Czechoslovak Chemical Communications, 1963 , vol. 28, p. 1419 - 1426 DOI:10.1135/cccc19631419