Rep:Mod:SPK2497
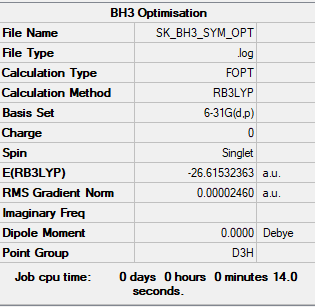
BH3
Method- B3LYP
Basis Set- 3-21G level
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000195 0.001800 YES RMS Displacement 0.000128 0.001200 YES
Link to the frequency file- Media:SK_BH3_FREQ.LOG
Low frequencies --- -0.2279 -0.0081 -0.0008 22.0037 22.0049 24.0346 Low frequencies --- 1163.1731 1213.2725 1213.2727
Optimised BH3 Molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1 | IR active? | type |
| 1163 | yes | symmetric bend |
| 1745 | yes | asymmetric bend |
| 1745 | yes | asymmetric bend |
| 3390 | no | symmetric stretch |
| 3543 | yes | asymmetric stretch |
| 3543 | yes | asymmetric stretch |
There are only 3 peaks in the vibrational spectrum, while there are 6 vibration because there are 2 sets of degenerate vibrations ( asymmetric bend and asymmetric stretch), 1 unique vibration ( symmetric bend) and a vibration which doesn't result in a change of dipole, therefore is not detected (symmetric stretch).
Molecular orbital Diagram
Reference- Hunt Research Group. Molecular Orbitals of BH3. Available from: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf. [ Accessed 2nd May 2019]
The main difference between the real and LCAO MOs is that in the real MOs, parts of orbitals with the same phase are combined together, whereas in LCAO they are separate. Overall, qualitative MO theory doesn't necessarily give us an accurate depiction of what real MOs look like although it is a useful tool.
Clear representation of all of the relevant MOs on the diagram. Good attempt at considering the differences between the approaches but the idea isn’t quite correct as the LCAOs are just drawn that way by convention as we draw each of the fragments. The overall MO shape itself should arise from you considering the relative in-phase and out-of-phase additions and cancellations from the fragments. Smf115 (talk) 22:29, 13 May 2019 (BST)
Association Energy of Ammonia Borane
E(NH3)= -56.56 AU
E(BH3)= -26.62 AU
E(NH3BH3)= -83.22 AU
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.04AU= -105.02 kJ/ mol - this implies that the B-N bond is a weak bond.
Correct calculation but sadly you rounded the energies too early (also to the wrong degree of accuracy) which arose in the wrong answer. No literature values are used to compare to your answer meaning that you have no evidence to conclude that it is a weaker bond. Smf115 (talk) 22:30, 13 May 2019 (BST)
NI3
Link to NI3 File- Media:NI3_OPT.LOG
Item Value Threshold Converged? Maximum Force 0.000061 0.000450 YES RMS Force 0.000037 0.000300 YES Maximum Displacement 0.000459 0.001800 YES RMS Displacement 0.000285 0.001200 YES
Optimised NI3 Molecule |
Optimised NI3 distance- 2.18398 Å
Overall, an ok effort at the first section but largely unfinished and the NH3, NH3BH3 and NI3 frequency calculations are missing. It would have been good to see some attempt at the project section. Smf115 (talk) 22:30, 13 May 2019 (BST)