Rep:Mod:SP123
NH3 Molecule
Optimisation
The molecule is ammonia.
The calculation method is RB3LYP.
The basis set is 6-31G(d.p).
The final energy is -56.55776873 a.u.
The RMS gradient is 0.00000485 a.u.
The point group of ammonia is C3V.
The optimized bond length is 1.01798 Angstroms and the optimized bond angle is 105.74115 degrees.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986260D-10
Optimization completed.
-- Stationary point found.
Ammonia molecule |
The optimisation file is linked to here
Molecular Vibrations
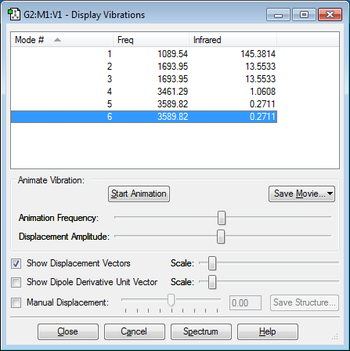
According to the 3N-6 rule I would expect there to be 6 modes of vibration.
Modes 2 and 3 are degenerate and modes 5 and 6 are also degenerate.
Modes 1,2 and 3 are bending vibrations and modes 4,5 and 6 are stretching vibrations.
Mode 4 is highly symmetric.
Mode 1 is the umbrella mode.
I would expect to see two bands in the experimental spectrum of gaseous ammonia and these bands are due to mode 1 of vibration and the other band corresponds to modes 2 and 3 of vibration which are degenerate. Band 4,5 and 6 will not appear in the spectrum because the intensity of the peaks are very low.
Charge Analysis
The charge on the nitrogen atom is -1.125 a.u. and the charge on the hydrogen atoms are 0.375 a.u..
I would expect the nitrogen to have a negative charge because nitrogen is more electronegative than hydrogen and hence strongly attracts the electron density.
H2 and N2 Molecules
H2 Molecule
Optimisation
The molecule is Hydrogen.
The calculation method is RB3LYP.
The basis set is 6-31G(d.p).
The final energy is -1.17853935 a.u.
The RMS gradient is 0.00003809 a.u.
The point group of hydrogen is D∞h.
The optimized bond length is 0.74289 Angstroms.
Item Value Threshold Converged?
Maximum Force 0.000066 0.000450 YES
RMS Force 0.000066 0.000300 YES
Maximum Displacement 0.000087 0.001800 YES
RMS Displacement 0.000123 0.001200 YES
Predicted change in Energy=-5.726834D-09
Optimization completed.
-- Stationary point found.
Hydrogen molecule |
The optimisation file is linked to here
Molecular Vibrations
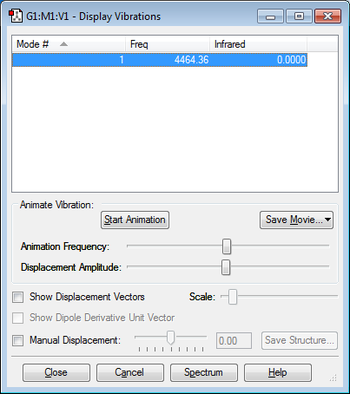
There is one vibrational mode which is predicted by the 3N-5 rule for linear molecules. This mode does not have a negative frequency. I would expect to see one band in the experimental spectrum for the hydrogen.
Charge analysis
The charge of both the hydrogen atoms in the molecule is 0.000 a.u. this is expected because the two hydrogen atoms have the same electronegativity.
N2 Molecule
Optimisation
The molecule is Nitrogen.
The calculation method is RB3LYP.
The basis set is 6-31G(d.p).
The final energy is -109.52412868 a.u.
The RMS gradient is 0.00000282 a.u.
The point group of nitrogen is ∞h.
The optimized bond length is 1.10550 Angstroms.
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-7.433914D-12
Optimization completed.
-- Stationary point found.
Nitrogen molecule |
The optimisation file is linked to here
Molecular Vibrations
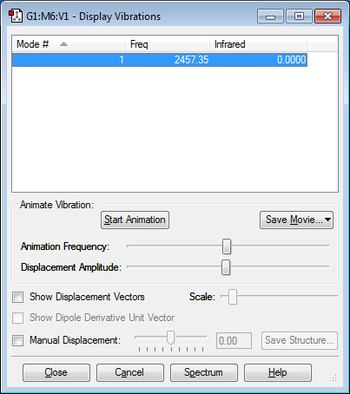
There is one vibrational mode as predicted by the 3N-5 rule for linear molecules. This mode does not have a negative frequency. I would expect to see one band in the experimental spectrum for nitrogen.
Charge analysis
The charge of both the nitrogen atoms in the molecule is 0.000 a.u. this is expected because the two nitrogen atoms have the same electronegativity.
Reaction energy for the Haber-Bosch process
E(NH3)=-56.55776873
2*E(NH3)=-113.1155375
E(N2)=-109.52412868
E(H2)=-1.17853935
3*E(H2)=-3.53561805
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.05579077 a.u.
ΔE= -146.48 KJmol-1
Therefore the enthalpy of the reaction is negative and therefore the reaction is exothermic and the products are more stable than the reactants.
SiH4 Molecule
Optimisation
The molecule is Silane.
The calculation method is RB3LYP.
The basis set is 6-31G(d.p).
The final energy is -291.88802747 a.u.
The RMS gradient is 0.00005848 a.u.
The point group of Silane is Td .
The optimized bond length is 1.48454 Angstroms and the bond angle is 109.47122 degrees.
Item Value Threshold Converged?
Maximum Force 0.000113 0.000450 YES
RMS Force 0.000061 0.000300 YES
Maximum Displacement 0.000589 0.001800 YES
RMS Displacement 0.000315 0.001200 YES
Predicted change in Energy=-1.333300D-07
Optimization completed.
-- Stationary point found.
Silane molecule |
The optimisation file is linked to here
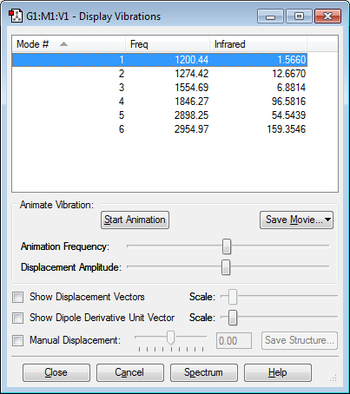
Molecular vibrations
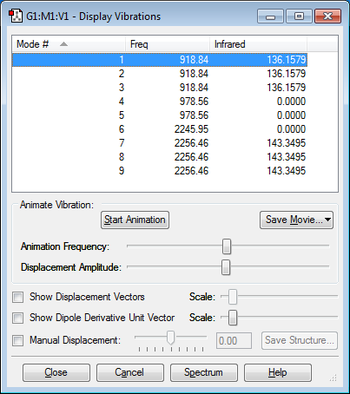
The display vibrations shows that overall silane has nine vibrational modes as predicted by the 3N-6 rule for non-linear molecules.
Vibrational modes 1, 2 and 3 are degenerate, vibrational modes 4 and 5 are also degenerate and vibrational modes 7,8 and 9 are also degenerate.
Vibrational modes 1,2,3,4 and 5 are all bending vibrations and the vibrational modes 6,7,8 and 9 are all stretching frequencies.
I would expect to see two bands on the experimental spectrum for silane the first band corresponds to modes 1,2 and 3 of vibration (as these three modes are degenerate) and the first band corresponds to the modes 7,8 and 9 of vibration (as these three bands are also degenerate). I would not expect to see bands on the spectrum corresponding to modes 4,5 and 6 of vibration as these modes have an intensity of 0.
Charge Analysis
The charge of the silicon atom is +0.630 a.u. and the charge of the hydrogen atoms are -0.157 a.u. and this therefore shows that the hydrogen atoms are more electronegative than the silicon atom and therefore more strongly attract the electron density.
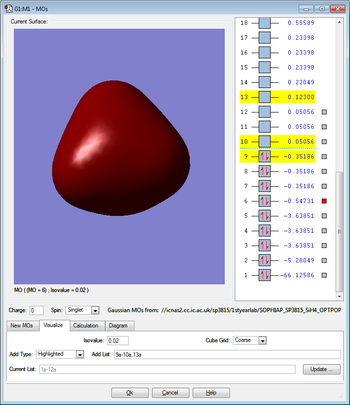
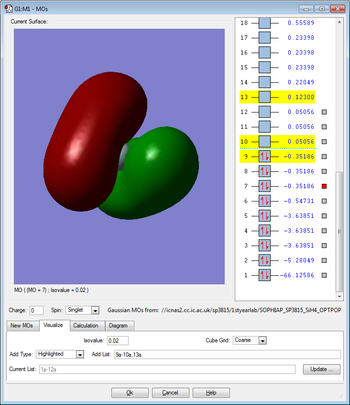
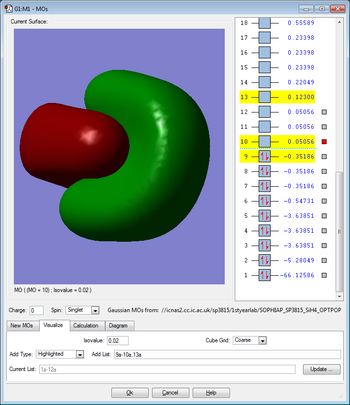
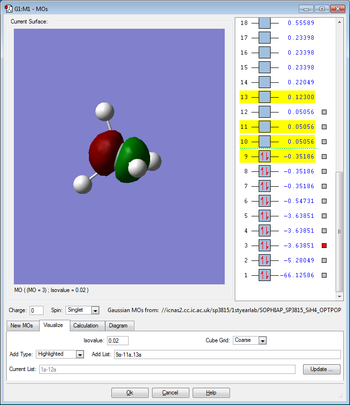
Molecular Orbitals
This molecular orbital is due to the overlap between the singly occupied 3s orbital of the silicon and the singly occupied 1s orbital of the hydrogen and therefore this molecular orbital is occupied by two electrons. This molecular orbital is bonding and also has sigma character. This molecular orbital is in the HOMO/LUMO region of energy.
This molecular orbital is due to the overlap between the singly occupied 3p orbital of the silicon and the singly occupied 1s orbital of the hydrogen and therefore this orbital is occupied by two electrons. There are three degenerate molecular orbitals of this type and this is due to the three degenerate 3p orbitals in the silicon. This molecular orbital is bonding and has sigma character. This molecular orbital is also the HOMO. This molecular orbital is in the HOMO/LUMO region of energy.
This molecular orbital is due to the overlap between the singly occupied 3p orbital of the silicon and the singly occupied 1s orbital of the hydrogen, however, this orbital is unoccupied. There are three degenerate orbitals of this type and this is again due to the three degenerate 3p orbitals in the silicon. This molecular orbital is anti-bonding and has sigma character. This molecular orbital is in the HOMO/LUMO region of energy.
This orbital is the 2s orbital of the silicon which is occupied by two electrons. This orbital is non-bonding because there is no overlap between the 2s orbital of the silicon and the 1s orbital of the hydrogen. This atomic orbital is deep in energy.
This orbital is the 2p orbital of the silicon which is occupied by two electrons. This orbital is non-bonding because there is no overlap between the 2p orbital of the silicon and the 1s orbital of the hydrogen. There are three degenerate orbitals of this type in the silicon atom. This atomic orbital is deep in energy.
COH2 Molecule
Optimisation
The molecule is Methanal.
The calculation method is RB3LYP.
The basis set is 6-31G(d.p).
The final energy is -114.50319936 a.u.
The RMS gradient is 0.00000912 a.u.
The point group of Methanal is C2v .
The optimized C=O bond length is 1.20667 and the optimized C-H bond length is 1.11044 Angstroms and the HCO bond angle is 122.39350 degrees.
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000032 0.001800 YES
RMS Displacement 0.000018 0.001200 YES
Predicted change in Energy=-4.751840D-10
Optimization completed.
-- Stationary point found.
Methanal molecule |
The optimisation file is linked to here
Molecular vibrations
The display vibrations shows that the methanal molecule has six modes of vibration which is predicted by the 3N-6 rule for non-linear molecules.
Modes 1 and 2 of vibration are bending vibrations of the C-H bonds only.
Modes 3 and 4 of vibration are bending vibrations of the C-H bonds and the stretching of the C=O bond.
Mode 5 of vibration is the symmetric stretching vibrations of the C-H bonds only.
Mode 6 of vibration is the asymmetric stretching vibrations of the C-H bonds only.
None of the modes of vibration are degenerate and therefore I would expect to see six bands in the experimental spectrum for methanal.
I would expect to see five bands in the experimental spectrum for methanal and these five bands correspond to modes 2, 3, 4, 5 and 6 of vibrations. I would not expect to see a band corresponding to mode 1 of vibration as this mode has a very small intensity.
Charge Analysis
The charge analysis of methanal shows that the charge on the oxygen atom is -0.494 a.u., the charge of the carbon atom is 0.221 a.u. and the charge of the hydrogen atom is 0.137 a.u.
This therefore shows that the oxygen atom is more electronegative than the carbon atom and that the hydrogen atoms are more electronegative than the carbon atom and therefore attracts electron density more strongly.
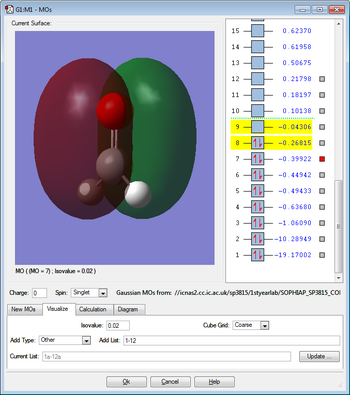
Molecular Orbitals
This molecular orbital is due to the overlap between the singly occupied carbon 2p orbital and the singly occupied oxygen 2p orbital and therefore this molecular orbital is occupied by two electrons. These two p orbitals are parallel to each other. This molecular orbital is bonding an has pi character.
This molecular orbital is due to the overlap between the singly occupied carbon 2p orbital and the singly occupied oxygen 2p orbital and therefore this molecular orbital is occupied by two electrons. The two p orbitals overlap head on and therefore the molecular orbital is bonding and has sigma character. There is also some pi character in C-H bonds due to partial overlap between the carbon 2p orbital and the 1s hydrogen orbital.