Rep:Mod:SML
Experiment 1C, Synthetic Modelling Lab, Sean Linsdall.
Molecular Mechanics
The MM2 molecular force field was developed by Normal Allinger in 1977, for the conformational analysis of small organic molecules and was designed to calculate the equilibrium geometry of the target molecule as precisely as possible. [1] This force field will therefore be be suitable for all of the molecules which are going to be investigated below. The MMFF94 molecular force field was developed by the Merck research laboratory in 1996 and was based on the MM3 molecular force field, during it's development the aim was to achieve MM3 like accuracy for small molecules and to be able to perform on large organic molecules too e.g. proteins etc. [2] For this reason the MM2 molecular force field has been used to optimism all of the structures before they were submitted to Gaussian for calculations.
Cyclopentadiene Derivatives
Cyclopentadiene Dimers
| Parameter | exo-dimer (1) (kcal/mol) | endo-dimer (2) (kcal/mol) |
|---|---|---|
| Stretching energy | 1.2851 | 1.2509 |
| Bending energy | 20.5805 | 20.8476 |
| Torsional energy | 7.6555 | 9.5109 |
| Van der Waals energy | 4.2334 | 4.3201 |
| Total energy | 31.8764 | 33.9975 |
From the above calculation it can be seen that the exo- form of the cyclopentadiene dimer (1) is thermodynamically more stable as it has a lower total energy. However it is known that the endo- cyclopentadiene dimer (2) is almost exclusively formed, despite it being thermodynamically unstable with respect to 1. This occurs because 1 is the kinetically more stable isomer as the transition state via which it proceeds is lower in energy than 2. [3]
Hydrogentaion of Cyclopentadiene Dimers
| Parameter | molecule 3 (kcal/mol) | molecule 4 (kcal/mol) |
|---|---|---|
| Stretching energy | 1.2777 | 1.0964 |
| Bending energy | 19.8586 | 14.5242 |
| Torsional energy | 10.8111 | 12.4975 |
| Van der Waals energy | 5.6328 | 4.5126 |
| Total energy | 35.6850 | 31.1520 |
Hydrogenated cyclopentadiene dimer 4 is thermodynamically more stable than than 3 as it has an overall lower total energy. Stretching, bending, and Van der Waals energy are all lower in 3, the largest energy difference is in the bending energy where 3 is 5.3344 kcal/mol lower in energy. However 4 is more torsionally strained than 3 by 1.6862 kcal/mol, which is due to the double bond being in a five membered ring opposed to a 6 membered ring.
Taxol
Taxol Isomers
| Parameter | Taxol (9a) Twist Boat (kcal/mol) | Taxol (9b) Chair (kcal/mol) | Taxol (10a) Twist boat (kcal/mol) | Taxol (10b) Chair (kcal/mol) |
|---|---|---|---|---|
| Stretching energy | 2.9480 | 2.7001 | 2.8327 | 2.6205 |
| Bending energy | 17.2119 | 15.8941 | 12.9782 | 11.3391 |
| Torsional energy | 21.2838 | 20.2991 | 22.9840 | 19.6722 |
| Van der Waals energy | 14.5057 | 13.2894 | 14.1197 | 12.8721 |
| Total energy | 53.3043 | 49.5288 | 49.5132 | 42.6828 |
As can be seen above 10 is the most stable form of taxol as it has the lower total energy compared to 9. Due to 9b being significantly higher in energy than 10b (6.846 kcal/mol) it is expected that 9b will convert to 10b if left to stand, this is an example of atropisomerism, and consequently 10b is the more stable antropisomer.
However both 9 and 10 can be found in different coformations, the lowest two energy forms are illustrated above (twist boat and chair). The chair conformations, 9b and 10b, are the lowest energy forms for 9 and 10 by a value of;
- 9 = 3.7755 kcal/mol
- 10 = 6.8304 kcal/mol
This agrees with the general model of cyclohexane where the chair conformation is significantly lower in energy than the twist boat form.
The alkene in both 9 and 10 reacts slowly because it is bridgehead alkene bond, which confers significant stability to the double bond hence decreasing its reactivity. Wilhelm and Schleyer proposed a method for the calculation of alkene strain; subtracting the total strain energy of the parent hydrocarbon from the total strain energy of the alkene, both in their most stable conformation.[4] If this method is applied to 10b and its parent alkane it gives a value for the alkene strain of 9.2363 kcal/mol. This shows that 10b is significantly less strained than the parent alkane, therefore it will react very slowly as the reaction of is not thermodynamically advantageous.
Taxol derivatives
| Parameter | Taxol derivative (17) (kcal/mol) | Taxol derivative (18) (kcal/mol) |
|---|---|---|
| Stretching energy | 5.9240 | 5.3650 |
| Bending energy | 24.2534 | 20.2627 |
| Torsional energy | 27.2567 | 22.5545 |
| Van der Waals energy | 19.8893 | 17.4067 |
| Total energy | 76.4364 | 64.3641 |
Taxol derivative 18 is the more stable isomer out of 17 and 18 due to it having the lowest total energy. This agrees with the above section in which 10 is more stable than 9, this is what is expected as 17 and 18 are derivatives of 9 and 10 . However it has been observed that the cyclohexane ring in 18 does not form a perfect chair, this is due to the steric clash between the cyclohexane carbon atoms and the sulfur containing ring. 17 has been calculated in the twist boat form.
NMR Analysis
 |
 |
 |
 |
1H NMR Analysis
| Environments | Literature [5] | Computed | |||
|---|---|---|---|---|---|
| Chemical shift (δ) | Multiplicity | Splitting | Chemical shift (δ) | Multiplicity | |
| 29 | 5.21 | 1 | m | 6.00 | 1 |
| 24, 27, 36 - 39 | 3.00 - 2.70 | 6 | m | 2.47 - 2.93 | 6 |
| 50 - 53 | 2.70 - 2.35 | 4 | m | 3.14 - 2.93 | 4 |
| 41 - 46 | 2.20 - 1.70 | 6 | m | 2.34 - 1.22 | 6 |
| 40 | 1.58 | 1 | t, J= 5.4 Hz | 1.58 | 1 |
| 25, 26, 28 | 1.50 - 1.20 | 3 | m | 1.85 - 2.00 | 3 |
| 47 - 49 | 1.1 | 3 | s | 1.48 (0.62 - 2.34) | 3 |
| 33 - 35 | 1.07 | 3 | s | 1.30 (0.95 - 1.65) | 3 |
| 30 - 32 | 1.03 | 3 | s | 1.06 (0.95 - 1.16) | 3 |
| Environments | Literature [5] | Computed | |||
|---|---|---|---|---|---|
| Chemical shift (δ) | Multiplicity | Splitting | Chemical shift (δ) | Multiplicity | |
| 29 | 4.84 | 1 | dd (J=7.2, 4.7 Hz) | 5.54 | 1 |
| 50 - 53 | 3.40 - 3.10 | 4 | m | 2.98 - 3.45 | 4 |
| 40 | 2.99 | 1 | dd (J=6.8, 5.2 Hz) | 2.68 | 1 |
| 24 - 31, 38, 39, 44 - 48 | 2.80 - 1.35 | 14 | m | 1.05 - 3.27 | 14 |
| 35 - 37 | 1.38 | 3 | s | 1.15 (0.71 - 1.52) | 3 |
| 41 - 43 | 1.25 | 3 | s | 1.11 (0.97 - 1.25) | 3 |
| 32 - 34 | 1.10 | 3 | s | 1.07 (0.57 - 1.56) | 3 |
| 49 | 1.00 - 0.80 | 1 | m | 0.89 | 1 |
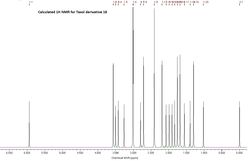
From the 1H NMR for 17 and 18 it can be seen that there is a reasonable agreement between the calculated chemical shift values and those reported by Paquette et al. [5] However there is a greater variation from the literature values for the chemical in 17 than there is in 18. This could be because 18 is in the stable chair conformation, whereas 17 is a less energetically stable twist boat formation. The greatest variation in the chemical shifts will between the hydrogen's which are part of the cyclohexane ring (H#41-46) and also the three hydrogen's on the methyl group attached to the cyclohexane ring (H#47-49). This is seen in 17 as there is a significant difference in width of the 14H multiplet, which is much larger for the calculated values of 17 than those reported.
13C NMR Analysis
| Environments | 12 | 4 | 5 | 14 | 10 | 3 | 13 | 6 | 15 | 22 | 23 | 17 | 11 | 18 | 9 | 2 | 7 | 1 | 16 | 8 |
| Literature δ [5] | 211.49 | 148.72 | 120.9 | 74.61 | 60.53 | 51.30 | 50.94 | 45.53 | 43.28 | 40.82 | 38.73 | 36.78 | 35.47 | 30.84 | 30.00 | 25.56 | 25.35 | 22.21 | 21.39 | 19.83 |
| Computed δ | 213.05 | 148.91 | 120.22 | 93.27 | 65.93 | 55.02 | 55.02 | 49.67 | 48.11 | 45.80 | 44.15 | 41.35 | 38.73 | 33.72 | 32.58 | 28.41 | 26.51 | 24.57 | 24.17 | 22.58 |
| Environments | 11 | 4 | 5 | 16 | 13 | 12 | 6 | 2 | 23 | 7 | 22 | 17 | 19 | 10 | 1 | 15 | 3 | 9 | 18 | 8 |
| Literature [5] δ | 218.79 | 144.63 | 125.33 | 72.88 | 56.19 | 52.52 | 48.50 | 46.80 | 45.76 | 39.80 | 38.83 | 35.85 | 32.66 | 28.79 | 28.29 | 26.88 | 25.66 | 23.86 | 20.96 | 18.71 |
| Computed δ | 214.46 | 149.37 | 116.91 | 91.27 | 64.54 | 60.33 | 54.01 | 52.86 | 47.63 | 46.63 | 44.19 | 40.07 | 33.19 | 32.87 | 31.46 | 30.69 | 27.36 | 26.87 | 22.05 | 21.57 |
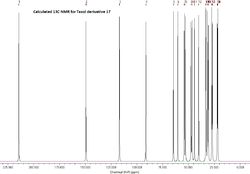
From the calculated 13C NMR it can be seen that generally there is a close agreement between the calculated values and the literature values, as all of the calculated values are within approximately 5ppm of those reported by Paquette et al.[5] There is one significant difference between the calculated and reported NMR values (highlighted in bold) this is from the carbon with the two sulfur atoms attached to it and is due to spin-orbit coupling errors, which are particularly prevalent when carbons are attached to 'heavy' atoms (halogens and sulfur especially). The difference between the literature value and that calculated is an average of 18.53 ppm, as the carbon is attached to two sulfur atoms there will be two lots of spin-orbit coupling errors, if we assume this is an additive effect then spin-orbit coupling correction for C-S is -9.26 ppm.
Taxol derivative 17 NMR calculations; DOI:10042/25313
Taxol derivative 18 NMR calulations; DOI:10042/25400
Energetic Analysis
 |
 |
The sum of the electronic and thermal free energy is equal to the Gibbs Free Energy as;
Therefore the Gibbs Free energy values for the Taxol derivatives are;
- Taxol derivative 17 = -1651.438 Hartree
- = - 4.33585 x106 kJ/mol
- Taxol derivative 18 = -1651.464 Hartree
- = - 4.33592 x106 kJ/mol
Meaning that 18 has the lower Gibbs free energy by 70 kJ/mol, this agrees with the conformational analysis calculations above where it was found that 18 was 12.0723 kcal/mol lower in energy than 17, which is the equivalent to 50.5 kJ/mol.
Crystal Structures
| Species 21 crystal structure | Species 23 crystal structure | ||||||
|---|---|---|---|---|---|---|---|
|
|
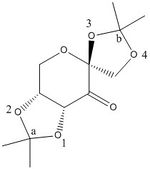
Structure 21
The O-C bond lengths at anomeric centre b are distinctly different (0.141 nm and 0.146 nm). This is due to the anomeric effect, at b each of the O-C bonds has a different bond anti-periplanar to it, O3-C has a C-C bond anti-periplanar to it so there will be donation from the σC-C bonding orbital into the σ*O3-C antibonding orbital. This increases the electron density in the σ*O3-C orbital and causes the O3-C bond to weaken therefore becoming longer. Whereas the O4-C bond only has a σH-C bond ani-periplanar to it, so there is less donation into the σ*O4-C orbital, hence why the O3-C bond is 5 pm longer that the O4-C bond.
The bond lengths at anomeric centre a are much closer in length and only differ by 1 pm as both of the O-C bonds are anti-clinal to the C-C bonds on the cyclohexane ring. Consequently there will be not be any donation from the σC-C bonding orbital into the σ*O-C antibonding orbital, due to them not being in an anti-periplanar reltionship.
Another interesting feature of the crystal structure of 21 is that the two dioxolane rings are found to be in different conformations. The ring which has anomeric structure a in it is arranged in a half-chair conformation, whereas the ring with anomeric center b in it is arranged in an envelope conformation. This could be due to pyran oxygen causing steric repulsion to oxygen 3 and hence making the envelope conformation more stable.
Structure 23
In structure 23 the two tBu group at the open face of the molecule experience an attractive relationship to each other as the maximum Van der Waal attractive distance for two hydrogen's is 0.240 nm. The distance between the two closest H's on the tBu group is 0.258 nm and 0.208 nm, the hydrogens which are 0.258 nm apart will experience a greater attraction that the two which are 0.208 nm apart. This is due to Van der Waals forces between hydrogen's start to become repulsive when the hydrogen atoms are 0.210 nm apart. As the Van der Waal forces between the tBu hydrogen's which face each other are in the attractive range (around 0.240 nm), this will convey stability to the molecule. [6]
There is also a strongly attractive interaction between the hydrogen between the two tBu groups on the phenyl ring (H#7) and two of the carbons of the para tBu groups (C#57 and C#61). The Van der Waals distance of maximum attraction between a C - H is 0.270 nm, and the distance between H#7 and C#61 is 0.271 nm, which means there is a very strong interaction between these the hydrogen and the tBu group which will hold the para tBu group in that position.
Calculated NMR
Trans-β-methyl styrene epoxide
 |
 |
 |
 |
1H NMR
| Environment | Literature[7] | Computed (R,R) | |||||
|---|---|---|---|---|---|---|---|
| Chemical shift (δ) | Multiplicity | Splitting | Chemical shift (δ) | Multiplicity | Chemical shift (δ) | Multiplicity | |
| 11 - 15 | 7.25 | 5.11 | m | 6.98 (6.81 - 7.15) | 5 | 7.14 (7.21 - 7.07) | 5 |
| 16 | 3.5 | 0.97 | s | 4.26 | 1 | 4.35 | 1 |
| 17 | 2.95 | 0.97 | m | 3.80 | 1 | 3.95 | 1 |
| 18 - 20 | 1.35 | 4.75 | s | 1.70 (1.27 - 2.12) | 3 | 1.75 (1.32 - 2.19) | 3 |
As can be seen from the 1H NMR data above there is a close agreement between the (R,R) and (S,S) enantiomers and the literature values as they both follow the same general trend. The greatest difference between the calculated values and those reported in the literature is for environments 16 and 17 which are the two hydrogen's attached to the epoxide carbons. There is a slight difference in the computed NMR values between the (R,R) and the (S,S) enantiomers, which again is most significant for environment 16 and 17 which is due to the differences in stereochemistry.
13C NMR
| Environment | Literature[7] | Computed (R,R) | ||||
|---|---|---|---|---|---|---|
| Chemical Shift (δ) | Multiplicity | Chemical Shift (δ) | Multiplicity | Chemical Shift (δ) | Multiplicity | |
| 5 | 137.9 | 1 | 142.76 | 1 | 143.64 | 1 |
| 1 | 128.5 | 1 | 134.35 | 1 | 135.14 | 1 |
| 2, 3, 4, | 128.1 | 3 | 132.74 | 3 | 133.54 | 3 |
| 6 | 125.7 | 1 | 126.86 | 1 | 127.84 | 1 |
| 8 | 59.6 | 1 | 89.5 | 1 | 91.84 | 1 |
| 7 | 59.1 | 1 | 85.48 | 1 | 86.97 | 1 |
| 9 | 18.0 | 1 | 35.66 | 1 | 36.02 | 1 |
From the 13C NMR it can be seen that there is a close agreement between the literature and computed values for both enantiomers for the 6 aromatic carbons (environments 1-6), all of which are in the expected aromatic region for 13C NMR. However there is significant variation between the chemical shifts for the epoxide (8,7) and methyl (9) carbons from the literature values, which indicated that the conformation of trans-β-methyl styrene was not the lowest energy conformation.
Trans-β-methylstyrene epoxide NMR calculations; (R,R) = DOI:10042/25488 , (S,S) = DOI:10042/25658
Trans-stilbene epoxide
 |
 |
 |
 |
1H NMR
| Environment | Literature[8] | Computed (R,R) | |||||
|---|---|---|---|---|---|---|---|
| Chemical shift (δ) | Multiplicity | Splitting | Chemical shift (δ) | Multiplicity | Chemical shift (δ) | Multiplicity | |
| Aromatic H's | 7.30 - 7.38 | 10 | m | 7.09 - 7.29 | 10 | 7.13 - 7.47 | 10 |
| 21, 22 | 3.87 | 2 | s | 4.64 | 2 | 5.16 (5.05 - 5.24) | 2 |
From the 1H NMR for trans-stilbene there is a close agreement between the computed and literature values for the aromatic hydrogen's, as they are all in the chemical shift range of 7.00 - 7.40 which is what is expected of aromatic hydrogen's. However again there is a difference in the chemical shift values between the literature and the computed hydrogen's which are connected to the carbons of the epoxide motif (21 and 22). This could again be due to the lowest energy form of trans-stilbene not being used for the calculation.
13C NMR
| Environment | Literature[8] | Computed (R,R) | ||||
|---|---|---|---|---|---|---|
| Chemical Shift (δ) | Multiplicity | Chemical Shift (δ) | Multiplicity | Chemical Shift (δ) | Multiplicity | |
| 5, 9 | 137.1 | 2 | 143.81 | 2 | 145.16 | 2 |
| 1, 3, 11, 13 | 128.5 | 4 | 134.47 | 4 | 134.42 | 4 |
| 2, 12 | 128.3 | 2 | 133.72 | 2 | 133.74 | 2 |
| 4, 6, 10, 14 | 125.5 | 4 | 130.5 | 4 | 132.63 | 4 |
| 7, 8 | 62.8 | 2 | 92.7 | 2 | 89.53 | 2 |
As can be seen from the 13C NMR there is a close agreement between the literature and computed values for the aromatic carbons (environments 1-6, 9-14), all of which are in the expected aromatic region for 13C NMR. However there is significant variation between the chemical shifts for the epoxide (7,8) from the literature values, which indicates that the conformation of trans-β-methyl styrene was not the lowest energy conformation.
Trans-stilbene epoxide NMR calculations; (R,R) = DOI:10042/25503 , (S,S) = DOI:10042/25646
Calculated Chiroptical properties
The chiroptical optical properties of the alkenes were calculated using optical rotatory method, which calculates the optical rotation of the molecule at a specific wavelength of light (589 nm and 365nm), these have been reported below for the trans-β-methylstyrene epoxide and trans-stilbene epoxide, and the calculated values have been compared to previously established literature values.
Trans-β-methylstyrene epoxide
| Wavelength | Literature (R,R)[9] | Computed (R,R) | |
|---|---|---|---|
| 589 nm | 49.2 ° | 76.54 ° | -76.54 ° |
| 365 nm | n/a | 202.59 ° | -202.59 ° |
The computed optical rotations for (R,R) and (S,S) are equal in magnitude, but opposite in phase. This indicates for the two molecules calculated they were perfect enantiomers of each other, consequently the stereo-chemistry at both of the chiral centres are opposite in each of the enentiomers. However the optical rotation values for both enantiomers does not agree with the literature values, indicating that the structure of which the computed values were run was not in fact the lowest energy form.
Trans-β-methylstyrene epoxide optical rotation calculations; (R,R) = DOI:10042/25659
Trans-stilbene epoxide
| Wavelength | Literature (R,R)[10] | Computed (R,R) | |
|---|---|---|---|
| 589 nm | 239.2 ° | 233.77 ° | -233.77 ° |
| 365 nm | n/a | 840.54 ° | -840.54 ° |
The optical rotation values of the two enantiomers (R,R) and (S,S) are equal in magnitude but opposite in phase, so the two structures are the opposite enantiomeric forms of trans-stilbene epoxide. The literature value is close in magnitude to the computed value therefore the structure used is almost the lowest energy form of trans-stilbene epoxide, but as the optical rotations do not exactly match then the structure of trans-stilbene epoxide which was computed was not the lowest energy conformation. However there are several different values for the optical rotation of trans-stilbene epoxide which have been reported in the literature, meaning further study would be needed to determine the exact optical rotation of trans-stilbene epoxide
Trans-stilbene epoxide optical rotation calculations; (S,S) = DOI:10042/25662
Transition state properties
Shi Catalyst
Free energies (Hartree) for transition states of the Shi epoxidation of β-methyl styrene
| (R,R) series | (S,S) series | ||
|---|---|---|---|
| TS #1 | - 1343.022970 | TS #1 | - 1343.017942 |
| TS #2 | - 1343.019233 | TS #2 | - 1343.015603 |
| TS #3 | - 1343.029272 | TS #3 | - 1343.023766 |
| TS #4 | - 1343.032443 | TS #4 | - 1343.024742 |
The lowest energy transition state above is TS# 4 in the (R,R) series, which is (R,R) trans-stilbene with the phenyl group orientated exo to the fructose ring.
- Lowest E TS in (R,R) series (TS# 4) = - 1343.032443
- Lowest E TS in (S,S) series (TS# 4) = - 1343.024742
- ΔG = - 0.007701 Hartree
- ΔG = - 20.21898 kJ/mol
- ΔG = RT Ln(k)
- k = 3486.65
- , where x = enantiomeric excess
- x = 0.9997
The calculated enantiomeric excess of 99.997% is in favour of (R,R). As the rate constant was calculated for the equilibrium (S,S) --> (R,R) which gave a rate constant of almost 1, meaning that the equilibrium lies almost completely to the right, therefore (R,R) is in enantiomeric excess. This agrees with the literature value of 90% (R,R) in enantiomeric excess by Shi et al. [11]
Jacobsen Catalyst
Free energies (kcal/mol) for transition states of the Jacobsen epoxidation of cis-β-methyl styrene
| (S,R) series | (R,S) series | ||
|---|---|---|---|
| TS #1 | - 3383.259559 | TS #1 | - 3383.251060 |
| TS #2 | - 3383.253442 | TS #2 | - 3383.250270 |
The lowest energy transition state for the Jacobsen catalyst is TS# 1 in the (S,R) series, which is cis-β-methylstyrene where the phenyl group is orientated underneath the catalyst.
- Lowest E TS in (S,R) series (TS# 1) = - 3383.259559
- Lowest E TS in (R,S) series (TS# 1) = - 3383.251060
- ΔG = -0.008499 Hartree
- ΔG = -22.31412 kJ/mol
- ΔG = RT Ln(k)
- k = 8118.63
- , where x = enantiomeric excess
- x = 0.9999
The calculated enantiomeric excess is 99.997% in favour of (S,R). As the rate constant was calculated for the equilibrium (R,S) --> (S,R) which gave a rate constant of almost 1, meaning that the equilibrium lies almost completely to the right, therefore (S,R) is in enantiomeric excess. This agrees with the literature value of a 92% enantiomeric excess of (S,R) by Jacobsen et al. [12]
Non-covalent Interactions in the reaction transition state
Shi Catalyst
| Lowest Energy Shi Transition State TS#4 (R,R) | |||
|---|---|---|---|
|
As can be seen from the non covalent interactions illustrated for the Shi catalst there are several different interactions which hold the alkene in position whilst it is being epoxidised as well as providing stabilizing interactions to the transition state
- There is an attractive hydrogen bond like interaction between the dioxirane oxygen not involved in the epoxidation and two hydrogen's (H#17 - O#35 - H#33). The lone pair on the dioxirane oxygen will be placed so that there is an attractive force to the two hydrogen's. As these three atoms are almost linear (162.3°), this interaction is very important in holding the dioxirane group in the correct position so that epoxidation can occur.
- The phenyl group of the alkene will have a high density electron π cloud above and below it due to the aromatic system, causing this to be a δ- environment. H#19 is adjacent to the oxygen in the dioxolane ring, this will have an electron withdrawing effect causing the hydrogen to be acidic and consequently δ+. The δ+ hydrogen is located directly above the δ- phenyl group so there will be a strong attractive interaction between the two groups. This attraction will hold the alkene in place therefore contributing towards the transition state stability.
- H#56, on the methyl group of the alkene is in a position so that it able to form an attractive interaction with O#2, this will hold the alkene in the optimum position so that the epoxidation occurs at the lowest energy, hence contributing toward this transition state being the lowest in energy.
- The hydrogen attached to the CH of the alkene (H#37) is in a position which allows an attractive interaction with the hydrogen of the tBu group (H#28). These two hydrogen's are 0.202 nm apart, the distance for the maximum attraction between two hydrogen's is 0.240 nm, so there will be a strong Van der Waals attraction between the two which again conveys stability to the transition state. [6]
Jacobsen Catalyst
| Lowest Energy Jacobsen Transition State TS#1 (S,R) | |||
|---|---|---|---|
|
As can be seen from the non covalent interactions of the transition state for the epoxidation of β-methyl styrene with a Jacobsen catalyst there are lots of different non covalent interactions which hold the alkene in place whilst it is being epoxidised and also convey stability to the transition state.
- The phenyl ring of the alkene is aromatic so there will be a large π system above and below it which will be δ-, this is directly placed above two carbon atoms (C#33 and C#42) which are adjacent to the nitrogen and oxygen. As these two carbons are next to the electron withdrawing hetero-atoms they will become δ+, hence there will be an non covalent attraction between the phenyl ring and the two carbons.
- The phenyl ring of the alkene is approximately 0.350 nm above carbons (C#33, C#35 and C#42). The Van der Waal radius of carbon is 0.160 nm, so the distance where the maximum interaction will be felt between the two layers of carbons when they are 0.320 nm apart. As the layers are approximately 0.350 nm apart there will be a strong Van der Waals interaction between the two layers as seen by the non-covalent interactions. [6]
- There is a strong attraction between the hydrogen of the alkene and the hydrogen's on the tBu group (H#48 and H#69), these hydrogen's are 0.193 nm apart. The Van der Waal radius of hydrogen is 0.120 nm so the optimum attractive distance is 0.240 nm, however the distance between these two hydrogen's is a lot closer than the optimum distance yet there is still an attractive interaction as shown by the blue non covalent interaction. [6]
- Another strong non covalent interaction is between the chlorine atom and H#18, the distance between the two atoms is 0.294 nm, and the optimum Van der Waal distance is 0.285 nm. This will therefore be a strong interaction, and will keep the side of the catalyst on which the epoxide approaches free and as flat as possible, which will allow a greater interaction therefore lowering the energy of the transition state.
Electron topology in the reaction TS
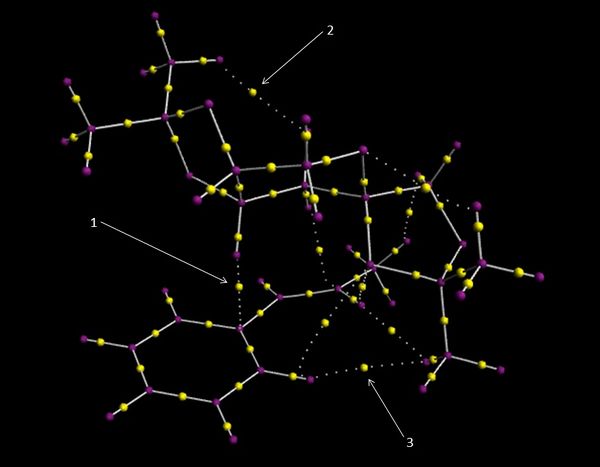
The electron topology for the transition state (TS# 4) of the epoxidation of (R,R) trans-β-methyl, with a Shi catalyst is shown above. The purple dots represent the atoms in the molecule and the yellow dots represent Bond Critical Points (BCP), which is an area where the electron density. If a yellow dot is on a solid line this represents a formal covalent bond, i.e. an area of high electron density, however if the yellow dot is not on a dotted line then this represents an area of high electron density which is a non-covalent interaction. In the electron topology above there are several areas which have strong non-covalent interactions:
- Interaction 1 is between H#19 which is part of the dioxolane ring and C#42 which is part of the phenyl ring of trans-β-methyl styrene. The hydrogen is part of a CH unit which is adjacent to a oxygen atom. This relationship will cause the hydrogen to become δ+ due to the electron withdrawing effects of the δ- oxygen atom. Whereas the carbon in the phenyl ring is going to be δ- due to the delocalized π electron cloud. The δ+ hydrogen and the δ- carbon will therefore be attracted to each other, which is seen from the area of high electron density between them.
- Interaction 2 is between H#33 which is part of the tBu group and O#35 which is the oxygen which will not become the epoxide in the dioxirane group. This area of high electron density is due to the δ- oxygen having a lone pair of electrons which are directed towards the tBu group, this forms an attraction with the hydrogen, which can be seen by the area of high electron density between the two atoms. The Bond Critical point for interaction 2 is closer to the hydrogen than the oxygen, this confirms that the interaction is due to the electro-negative oxygen being attracted to the electro-positive hydrogen.
- Interaction 3 is between two hydrogen atoms; H#26 which is on a tBu group and H#46 which is on the phenyl group these two atoms are 0.276 nm apart. The Van der Waals radius of a hydrogen atom is 0.120 nm, so the maximum attractive distance between two hydrogen's is 0.270 nm, therefore as there two hydrogen's are 0.276 nm there is going to be a strong Van der Waals interaction between the two of them. As the Bond Critical Point is at an equal distance from both atoms this confirms that it is a Van der Waal attractive force, and not one of the atoms is polarized and is attracting another oppositely polarized atom. [6]
New Candidates for Investigation
A potential new candidate for the above analysis is (R)-(+)-Pulegone, which can be epoxidised as shown below.
The epoxidised (R)-(+)-Pulegone has a reported literature optical rotation value of 26° at a wavelength of 596 nm. [13] The alkene ((R)-(+)-Pulegone) is available to purchase from sigma-aldrich.
References
- ↑ Norman Allinger, "Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms", J. Am. Chem. Soc., 1977, 99 (25), 8127-8134.DOI:10.1021/ja00467a001
- ↑ Thomas A. Halgren, "Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94", J. Comp. Chem., 1996, 17 (25), 490-519.DOI:<490::AID-JCC1>3.0.CO;2-P :10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P
- ↑ Pierluigi Caramella , Paolo Quadrelli , and Lucio Toma, "An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the Endo Dimerization of Cyclopentadiene", J. Am. Chem. Soc., 2002, 124 (7), 1130–1131.DOI:10.1021/ja016622h
- ↑ Wilhelm F. Maier and Paul Von Rague Schleyer, "Evaluation and prediction of the stability of bridgehead olefins", J. Am. Chem. Soc., 1981, 103 (8), 1891–1900.DOI:10.1021/ja00398a003
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Leo A. Paquette , Neil A. Pegg , Dana Toops , George D. Maynard and Robin D. Rogers, "[3.3] Sigmatropy within 1-vinyl-2-alkenyl-7,7-dimethyl-exo-norbornan-2-ols. The first atropselective oxyanionic Cope rearrangement", J. Am. Chem. Soc., 1990, 112 (1), 277-283.DOI:10.1021/ja00157a043
- ↑ 6.0 6.1 6.2 6.3 6.4 Manjeera Mantina , Adam C. Chamberlin , Rosendo Valero , Christopher J. Cramer and Donald G. Truhlar* "Consistent van der Waals Radii for the Whole Main Group", J. Phys. Chem. A., 2009, 113 (9), 5806-5812.DOI:10.1021/jp8111556 Cite error: Invalid
<ref>tag; name "VdW radii" defined multiple times with different content - ↑ 7.0 7.1 Devendra J. Vyas, Evgeny Larionov, Céline Besnard, Laure Guénée, and Clément Mazet* "Isomerization of Terminal Epoxides by a [Pd–H] Catalyst: A Combined Experimental and Theoretical Mechanistic Study", J. Am. Chem. Soc., 2013, 135 (16), 6177-6183.DOI:10.1021/ja400325w Cite error: Invalid
<ref>tag; name "epoxide NMR" defined multiple times with different content - ↑ 8.0 8.1 Li Jia, Ya-Na Wanga, Chao Qiana* & Xin-Zhi Chena "Nitrile-Promoted Alkene Epoxidation with Urea–Hydrogen Peroxide (UHP)", Syn. Comms., 2013, 43 (16),2256-2264.DOI:10.1080/00397911.2012.699578 Cite error: Invalid
<ref>tag; name "epoxide NMR2" defined multiple times with different content - ↑ V. Conte,F. Di Furia,G. Licini,G. Modena,G. Sbampato,G. Valle*"Enantioselective oxidation of β-hydroxythioethers. Synthesis of optically active alcohols and epoxides", Tetrahedron: Asym., 1991, 2(4),257-276.DOI:10.1016/S0957-4166(00)80048-8
- ↑ Scott E. Denmark* and Hayao Matsuhashi "Chiral Fluoro Ketones for Catalytic Asymmetric Epoxidation of Alkenes with Oxone", J. Org. Chem., 2002, 67(10),3479-3486.DOI:10.1021/jo020050h
- ↑ O. Andrea Wong , Bin Wang , Mei-Xin Zhao and Yian Shi *"Asymmetric Epoxidation Catalyzed by α,α-Dimethylmorpholinone Ketone. Methyl Group Effect on Spiro and Planar Transition States", J. Org. Chem., 2009, 74(16)6335-6338.DOI:10.1021/jo900739q
- ↑ Eric N. Jacobsen , Wei Zhang , Alexander R. Muci , James R. Ecker , Li Deng, "Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane", J. Org. Chem., 1991, 113(18)7063–7064.DOI:10.1021/ja00018a068
- ↑ Koon-Sin Ngo, Kung-Kai Cheung and Geoffrey D. Brown, "Synthesis of α-Corocalene", J. Chem. Res. (S), 1998, 113(18)80-81.DOI:10.1039/A705069K