Rep:Mod:SJCryer Module3 Project
Diels Alder
Introduction
The diels alder reaction is a pericyclic reaction (cycloaddition) involving two pie orbitals interacting to form two new sigma bonds. The orbital overlap of these pie clouds strongly effects the geometry of the attack and so of the product. For attack to occur in the first place though, the HOMO and LUMO must align/overlap and also be of the correct symmetry. If they are not of the correct symmetry, they cannot overlap and so the reaction cannot take place.
The first reaction under investigation is that between butadiene and ethylene:

Cycloaddition of Ethylene to Butadiene
Cis-butadiene was firstly drawn into ChemBio 3D as this is the easiest way to draw it. It was minimised in energy and structure using the MM2 minimisation to give a basic optimised structure. It was then opened in GaussView and optimised using the semi-emperical AM1 method. This also gave the molecule orbitals.
 |
 |
 |
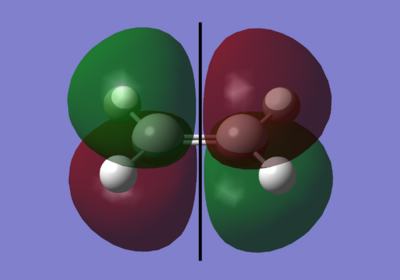 |
The black line down the centre of each image represents the reflection plane and is either symmetric or anti-symmetric.
As you can see here, for overlap to occur, it must be between the two symmetrical pie orbitals. this is the LUMO of the cis-butadiene and the HOMO of the ethylene.
Transition states
The first method to try and find the transition state was that of the QST2. The molecule's were set up as in the picture below and run under a HF/3-21g method and basis set. The result is next to it.
 |
 |
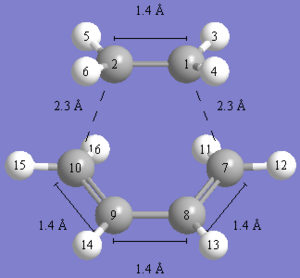
As you can see in the images above, a transition state was obtained, but the bonding in it seems rather strange. Is it very strange as you cannot even remove the double bond in the TS and GaussView does not seem to think it exists! That said, the estructure itself seems to be quite reasonable for a TS. It has a bond distance of about 2.2A for the terminal four carbons. This is a good estimate for a diels-alder TS and do would suggest it is the correct geometry.
From here, I tried to do a QST3 optimisation guessing the transition state in the middle. This went horrifically wrong and gave some strange thing with the terminal carbon bonds being around 1.5A which is more like product than a TS.
From here I thought it would be good to try and use the TS berny as I had some idea of what the TS now looked like. I drew the molecules in ChemBio3D and then orientated them into the guessed transition state structure. I then opened this in GausssView and ran a TS(berny) optimisation with frequency, calculating the force constant once at the start of the reaction. The method used this time was DFT 6-31g(d) basis set. This resulted in the transition state below:

This is highly likely to be the true transition state, with the forming bond being 2.27A. The vibrations were then looked at. There was only one imaginary frequency at -535cm-1. This corresponded to the bond formation between the ethylene and the butadiene. As shown in the picture below: the two reactants on the left and then moving together to give the transition state.

The bond formation is synchronous as both bonds are moving together and they are forming at the same time. This is confirms that the reactino is concerted which must be the case if it is to be classed as a pericyclic reaction. The lowest frequency after the imaginary one is at 135cm-1. When animated it shows all of the atomns in the molecule rocking. Becuase all of the molecules were doing this and the energy is so low, it suggests that it is a thermal vibration.
Below is an image showing the exact bond lengths of the diels alder product. A typical sp3 C-C bond length is abotu 1.54A whereas a typical sp2 C=C bond length is 1.33A. The C=C bond lengths are 1.38A and the C-C are about 1.41A. These lengths are somewhat in-between Sp2 and Sp3 bond lengths which means that in the transition state, the bond character is somewhere in-between. This is to be expected. The bonds between the two reactants are around 2.27A. This is a reasonable value for a diels-alder transition state, but is clearly a lot longer than an avergae C-C bond and this is because it clearly isn't a C-C bond yet. It has some sp3 bond character, but like the other bonds are less than sp3, this one is a lot greater than sp3. It shows how the 'sp2' 'sp3' valence model is jsut that... a model. When looking at molecular orbitals you can see that there is no 'exactly sp2/sp3' but often something inbetween which just corresponds to orbital overlap (or not as the case may be).
The Van de Waals radius is 1.7A for carbonDOI:10.1021/j100785a001 and so for there to be VdW interaction, the bond must be shorter than double this distance (3.4A). With the distance between considerably shorter than this (2.27A), it suggests that there is not just VdW interactions anymore, but the start of a sigma bond formation.
 |
NBO Analysis
I ran a full NBO analysis on the transition state so that the moelcular orbital interaction could be viewed. The most interesting orbital is the LUMO. Looking at the two symemtrical orbitals above (HOMO of ethylene/LUMO of butadiene), you can clearly see these have combined together in the transition state MO below. There is still a pie cloud on top of the ethylene like in the HOMO and the MO's under the butadiene have not changed from the LUMO. It is purely the electron density between the two molecules which have changed and these have combined to give a large (symmetric) area of bonding.

The other interesting MO that I saw was the HOMO-2. It is still a bonding orbital between the two reactants and shows a huge area of bonding beteen the two reactants. It looks to be between the HOMO of the ethylene and a different orbital on the butadiene.
 |
 |
Below is the HOMO, but this doesn't really show anything interesting.
 |
 |
Lastly is the HOMO-1. This is the most interesting after the LUMO. It looks to be the combination of the anti-symmetric orbitals (butadiene HOMO and ethylene LUMO). You can see it is anti-symmetric in nature:
 |
 |
To quote Woodward and Hoffman (1971)[1] "A reaction is allowed only when all the reactant bonding electrons are tranferred without symmetry-imposed barrier, into bonding orbitals of the products."
This reaction is classed as 'allowed' because the two symemtric orbitals have combined to give the LUMO and another symemtric orbital. The two anti-symmetric orbitals have also combined to give an anti-symemtric orbital
Diels-alder regioselectivity - Cyclohexa-1,3-diene & maleic anhydride
All of these calcualtions were done slightly differently to above. ChemBio3D was opened and a guessed transition state was drawn with the approximate bond length of 2.2A being made the 'optimum length'. This was then run with MOPAC under RM1 (which is more modern than AM1), to give a transition state. This was exported as a gaussian file and opened in GaussView. Then and optimisation and frequency were run using the DFT/6-31g(d) method and basis set and a TS(berny) optimisation with the force constant being calculated once.
The reaction sheme below shows how cyclohexa-1,3-diene & maleic anhydride combine to give both and endo and an exo product.
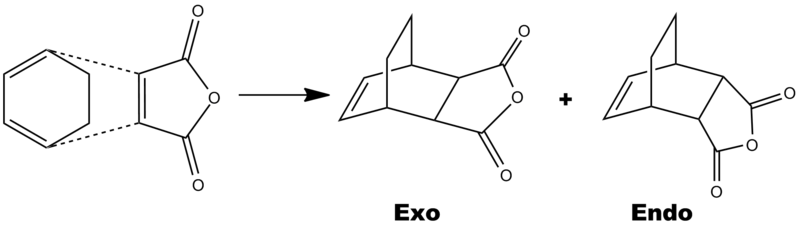 |
Below is a table showing the most crucial information about the endo and exo products. The most important is the energy. Although there is only a small difference (11.81KJmol-1), the endo product is the lowest in energy. Becuase it is the lowest energy transition state, it means that the kinetic endo product will predominately form in the reaction rather than the thermodynamically more stable exo product. If the transition state is lower in energy it means that the activation energy is lower and so this product forms.
| Product | Photo | Energy (HF) | Energy (KJmol-1) | Imaginary Freq. (cm-1) | Method and Basis-set |
| Endo |  |
-612.4954778 | -1.61E+06 | -447 | RB3LYP/6-31G |
| Exo |  |
-612.4909814 | -1.61E+06 | -448 | RB3LYP/6-31G |
Bond Lengths
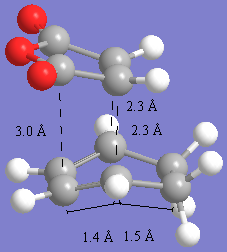
The images below show the transition state bond lengths to be 2.27A for endo and 2.29A for the exo product. This is again a reasonable bond length for diels-alder reactions. The through space distances are shown by measureing the distance between the closest atoms in the pretruding regions. These are around 3A for the endo product and 2.8A for the endo product. These are significantly close enough to take part in steric interactions and also secondary orbital overlap. The shorter bond for the endo product suggests there is slightly more orbital overlap in this molecule resulting in more bonding and this is one of the reasons that the endo product is the lower energy transition state. Anothe reason is to do with the steric interactions. In the exo form, the closest through space distance is to the hydrogens which are coming off the '-CH2-CH2' fragment. BEcuase this is a single bond and sp3 hybridised, the hydrogens stick out of the plane,r esulting in more of a steric clash with the oxygens on the maelic anhydride petrusion. With the endo form, the '-CH=CH-' part has sp2 flat hydrogens in the plane, and so the closest steric interaction is with the carbons which are further away (3A) than with the sp3 hydrogens on the exo (2.8A) resulting in less steric hinderance and a lower energy due to a shortening of the main TS bond.
 |
 |
MO's and Secondary orbital overlap
