Rep:Mod:SJCryer Module2 Project
Project
Introduction
The molecule under investigation is called Vanadyl acetylacetonate [V(O)(acac)2]. This is illustrated below. Is is of neutral charge. This is becuase the double bonded oxygen on the top are two x-type ligands, creating a +2 charge. The acac- (acetylacetonate anion) is an anion and so with two of these, the charge balances out to zero.
 |
The aim of the project is to look at how the charge distribution around the molecule is effected when changing the methyl groups on the acac with electron donating and electron withdrawing groups.
Literature[1] states that the vanadium complex will readily form different complexes with N-donor ligands which will come in underneithand occupy a site trans to the V=O bond. I aim to do a molecule NBO analysis of the various molecules and look to see if there are open orbitals to attack in the geometry trans to the V=O bond.
Initially when running calculations, I accidently put a charge of +2 on the molecule and ran all of the calculations (optimisation, NBO, etc.). Although this is actually wrong, it has given me the opportunity to compare the molecules as essentially I have ripped two electrons away from the system.
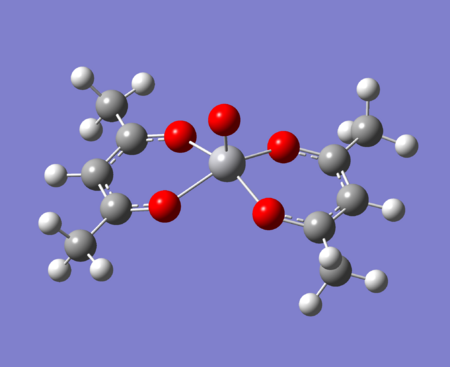
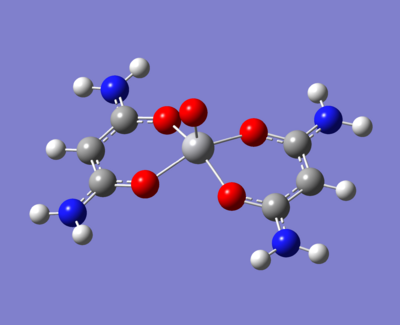
The there molecule under investigation are replacing the methyls on acac with:
- An electron donating group (NH2)
-The normal methyls
- An electron withdrawing group (fluorine)
These are as below:
 |
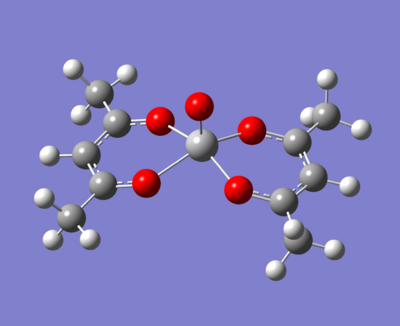 |
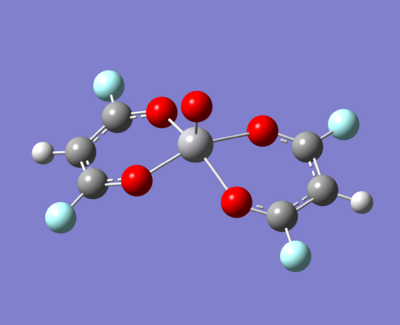 |
Optimisation
The first thing to do was optimise the structure of the molecule. This was initially done using chemdraw to get the molecule looking approximately correct (simple MM2, then moving the molecule around until it looked right). Then a loose optimisation was done with gaussian using basis set:
# opt=loose b3lyp/lanl2mb geom=connectivity DOI:1042/to-2681
From here a fine optimisation was done using the basis set:
# opt ub31yp/lanl2dz geom=connectivity int=ultrafine scf=conver=9 DOI:10042/to-2748
Notice the 'u' in front of the 'b3lyp' basis set. This is becuase the molecule has an un-even number of electrons and so it must be run as an unrestricted shell calculation. This menas that the spin up electrons are analysed completely seperate to the spin down electrons. This results in a set of 'alpha' and 'beta' molecular orbitals.
An NBO calculation was then done on the molecule using the basis set:
# ub3lyp/lanl2dz pop=nbo geom=connectivity DOI:10042/to-2751
A loose(DOI:10042/to-2759 ), fine(DOI:10042/to-2760 ) and NBO(DOI:10042/to-2762 ) calculation was then done for the nitrogen complex. Becuase the fluorine complex was so similar to the normal complex (without the methyl hydrogens), just a fine optimisation(DOI:10042/to-2764 ) and an NBO(DOI:10042/to-2762 ) calculatino were done.
The outcome of this is some interesting differences in the angle of the three systems. The planar 'rings' on each side of the molecule all have different angles.
 |
Here is a table of the angles:
| Complex | 1-2-3 angle |
| EDG (NH2) | 152.572 |
| acac | 150.838 |
| EWG (F) | 145.613 |
It would seem that the more electron donating the substituent, the larger the angle and the flatter the square planar complex is.
Charge distribution
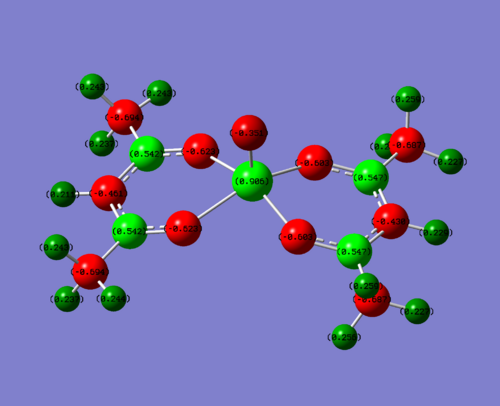
Below is the NBO analysis for the NBO charges on each atom in the vanadium complexes. This is calculated why looking at the charge on the molecule and then what the occupancy of the natural bonding orbitals are around this molecule. For example, if the occupancy is less than it should be (electrons have been withdrawn due to electronegative atom), then you would expect to see a positive charge.
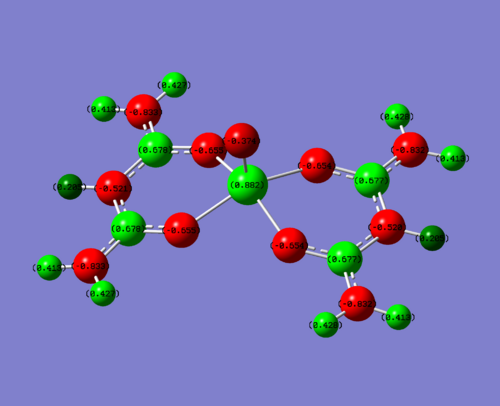 |
 |
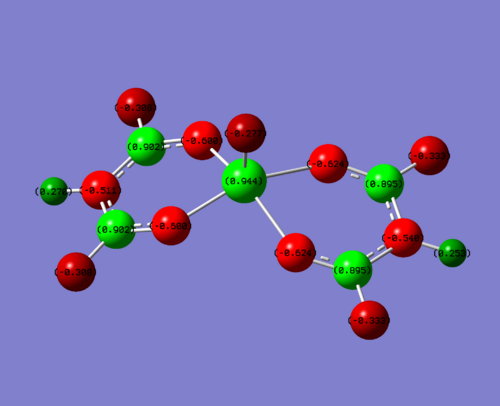 |
| Complex | NBO charge on V | NBO charge hetero | NBO charge on carbon attached to hetero | Charge on O of V=O |
| EDG (N) | +0.882 | N = -0.833 | 0.678 | -0.374 |
| Me | +0.906 | Me(C) = -0. | 0.542 | -0.351 |
| EWG (F) | +0.944 | F = -0.308 | 0.902 | -0.277 |
To clarify, the values stated above are for one of the four sections of the molecule. The charge is very similar throughout hte molecule, changing slightly in the 3rd d.p. This is due to the non-symmetrical nature of the molecule. For this discussion, this level of accuracy is not needed as it is purely a qualititive discussion.
So what can be deduced from this? Obviously, you would expect for the donating groups to donate electrons into the field creating more negative charge and the withdrawing groups to take electrons away, creating a more positive charge. This is indeed what you see, but how large is the effect?
The metal vanadium centre is three bonds away, and so is quite a large distance to be feeling the effects of the groups. But it does so. In the electron donating complex, the positive charge is 0.024 less than the initial acac complex. This is quite a significant difference (2.7% less) considering it is there bonds away and so this compositive electron donating effect from the N lone pair donating into the complex has had a large effect. The electron withdrawing fluorines have made an even larger difference, this time increasing the charge on the vanadium by 0.038 (4.2%). This is again a significant difference for three bonds through a molecule. This is due to fluorine being the most electronegative element on the periodic table.
One of the most interesting molecules to look at is the carbon attached to the electron hetero groups (NH2, Me, F). This displays some strange phenomena. It is positive for the acac complex (0.542) and ever more positive for the F complex (0.902). You would expect then for the electron donating NH2, it would be less positive. But it is actually more positive than the Me complex. So what is going on?
To explain this, a basic hypothesis can come about. Looking at the Me and F complexes temporarily, you can see that there is likely to be electron density being pulled through the framework in an inductive effect to give this positive charge on the metal centre. But it also looks like the bonds have highly ionic character in a very nice oscillating fashion. For example, the fluorine has a negative charge on it due to its electron withdrawing effects onto intself, the carbon next to it will have some kind of induced positive charge due to the negative nature of the one on fluorine. There is then another electron withdrawing oxygen atom next to this carbon which will have a slightly greater negative charge on it due to teh ionic positive anture of the carbon. The Vanadium centre then feels this effect as well, also beign slightly more positive.
Thanks to this oscilalting sinusoidal nature of the electronegative and positive atoms, you can easily create this polarising ionic effect to add to the inductive sigma framework. This hypothesis would explain the Me and F compelxes, but what about the more than expeted electropositive carbon attached to the nitrogen in the N complex?
Here we now have to take into consideration pie interations. In this situation, without the lone pair on nitrogen, you would just have an electronegative atom (nitrogen) on the carbon which would create an ionic and inductive effect through teh molecule very much like the fluorine. This is what is happening and is the reason for the high positive value of the NBO charge on the adjacent carbon.
This nitrogen does have a lone pair though and this can donate into the pie system it is adjacent to. The acac system is already a delocalised pie system and so the N lone pair donates electrons into this pie system which stretches all the way to the metal centre. This extra electron density in the molecule acts as the 'donating' effect, eventually causing the metal centre to have less positive charge than the Me and F complexes. So what we are seeing here is some kind of push pull effect. The lone pair pushing electrons into the system, but the electronegative nitrogen taking them back out. Clearly the pie interactions are a lot alrger adn so thsi is the dominating effect that is seen.
So how could this effect be investigated? What you need to do is to disrupt the system and see what kind of an effect there is. We already have one system with pie donation and it would be worth looking to see if another pie doning system acted in teh same way. The next thing to do would be to try and disrupt the nice sinusoidal pattern of positive and negative charge. Perhaps by putting in a sulpher atom in place on the four oxygens bonded onto the metal. Sulphur is much more diffuse and so would be a good molecule to use to see if this polarising effect was occuring. It would also be good to try and replace the carbon with another atom which is not so easily polarised, although finding something suitable could be difficult.
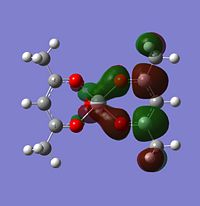
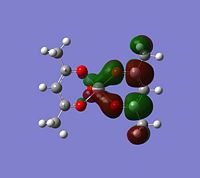
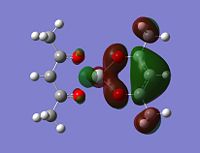
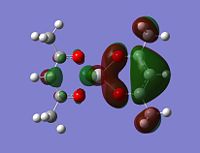
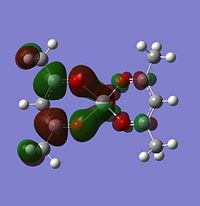
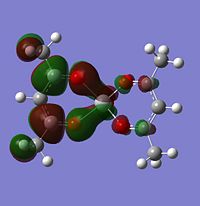
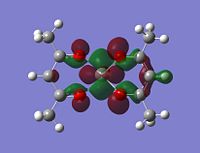
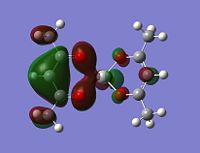
Molecular orbitals
The alpha has one more electron than the beta due to un-even number of electrons. Had to use un-restricted method.
- ↑ C. E. Housecroft and A. C. Sharpe - Prentice Hall publishers - 2004 - pages 604-605