Rep:Mod:SHMod1Lolcats
Computational Lab - Module 1 - Molecular Mechanics and Semi-Empirical Molecular Orbital Methods
Aim:
The aim of this experiment is to use molecular mechanics to predict geometry and regioselectivity of a number of reactions, with explanations as to deviations from predictions; the second aim is to use semi-empirical and DFT molecular orbital theory to investigate regioselectivity, neighbouring group participation (NGP) and spectroscopic simulation.
Molecular Mechanics Modelling
The molecular mechanics classical approach assumes that the energy of a system is made up of 5 different non interactive terms, these are the summations of all the bond stretches, bond angles, bond torsions, non-bonded repulsions and simple electrostatic interactions (where the stretches and angles come from a simple Hookes law potential to approximate a Morse curve). The molecular mechanics model attempts to minimise the energy by altering the bond lengths and angles. In this experiment ChemDrawPro is used to draw a simplified molecule which is then copied into ChemBio3D using the smiles format. By copying in the form of smiles ChemBio3D is forced to decide its own conformations for ring structures and will often (but not always) give the lowest energy conformation for each sub-structure in the molecule. Then the Allinger MM2 force field will be used to minimise the energy even further and analyse the energy of differing conformations of sub-structures to determine the lowest energy; the MM2 force field will also give some properties of the molecule based on how it optimised its geometry such as stretching, bending, torsion, Van der Waals and dipole-dipole interactions (dipole-dipole includes hydrogen bonding if there is any).
The Hydrogenation of Cyclopentadiene Dimer
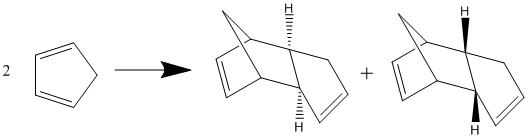
 |
 | |
| Total Energy (kcal/mol) | 34.03 | 31.88 |
The dimerisation of cyclopentadiene occurs spontaneously by a Diels-Alder reaction. This reaction specifically produces the endo dimer 2 over the exo dimer 1. This can be explained by the "endo rule" which shows that secondary orbital interactions favour the endo product over the exo product.
According to the MM2 optimisation data in table 1, the endo isomer has a higher energy by approximately 2 kcal/mol. This shows that the exo isomer is the thermodynamic product and the endo isomer is the kinetic product showing the reaction to be kinetically controlled.
In the hydrogenation of the endo dimer the MM2 optimisation data has shown that structure 4 is lower in energy then the 3 structure, this is due to the significantly reduced energy of bending and slightly reduced Van der Waals energy showing that product 4 is the thermodynamic product and product 3.
Stereochemistry of Nucleophilic additions to a Pyridinium Ring (NAD+ analogue)
In these reactions two pyridinium rings 5&7 undergo a stereospecific addition to form products 6&8.
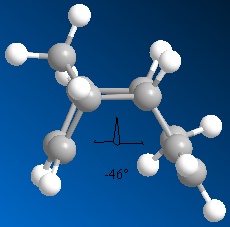
Seen as I copied molecule 5 on to chembio3D in the smiles format from chemdraw the conformation with the lowest energy was automatically selected although I tried to change the conformation of the 7 and 5 membered rings which only served to increase the energy of the molecule by a substatial amount; when the 7 membered ring was placed in the crown conformation the energy of the molecule quadrupled. The lowest energy conformation had the total energy of 36.8674 with a dihedral angle of the 7 membered ring at 9.0598o. Most of the energy contributions come from the bending and Van der Waals energies showing that the molecule has significant intermolecular interactions providing stability.
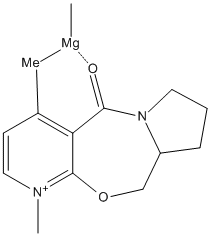
In this reaction a grignard reagent nucleophilically attacks the benzene ring to give a the methyl aligned with the carbonyl. Unfortunatly ChemBio3D does not have magnesium as a registered compound and so will not give an optimisation as to the placement of the magnesium. However according to a literature example of an stereospecific reaction of a grignard with a pyridinium ring[1] the highly electropositive magnesium coordinates to the carbonyl oxygen, thus fixing the position of addition of the methyl group onto the pyridinium, as shown by the mechanism (shown).
I also copied molecule 7 on to chembio3D in the smiles format from chemdraw so the conformation with the lowest energy was automatically selected again I tried to change the conformation of the 7 membered ring which only served to increase the energy of the molecule by a substatial amount. The lowest energy conformation had the total energy of 56.8913 with a dihedral angle of the 7 membered ring at 6.05o.Again most of the energy contributions come from the bending and Van der Waals energies showing that the molecule has significant intermolecular interactions providing stability.
The mechanism of stereospecific nucleopilic addition to pyridinium 7 is very different to this, according to the MM2 optimisation the nucleophilic nitrogen on the reactant is repelled by the electronegative carbonyl group and so attacks away from the carbonyl and so attacks from the top face to give the largest distance between the nitrogen and oxygen.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Atropisomerism occurs when a steric barrier prevents rotation around a bond and allows for the isolation of specific molecular conformations. In this case the carbonyl group can either be facing up or down.
Using the MM2 optimisation the carbonyl pointing up isomer is the lowest in energy by about 15 kcal/mol showing that the isomer with carbonyl pointing up is the most stable isomer.
The reason why the alkene in this molecule reacts very slowly is due to a phenomenon called hyperstabability[2], this occurs in ring systems with a single double bond where the strain of the alkene is actually less then the strain of the normal hydrocarbon, this therefore decreases reactivity of the double bond due to its so called "bridgehead" location.
Modelling using Semi-empirical Molecular Orbital Theory
The molecular mechanics approach has a number of limitations, these were shown most when the "endo rule" could not be understood by the MM2 method due to secondary orbital interactions. In the next part the electronic aspects of reactivity are shown to be just as useful to show how electrons influence bonds and can then be used to derive spectroscopic properties.
Regioselective Addition of Dichlorocarbene


From the vibrational analysis you can see that the large Chlorine substituent prevents vibration, or at least decreases the size of the vibrations of the nearest ring, the substituents on the opposite ring vibrate the most.
 |
 |
 |
 |
 |
Structure based Mini project using DFT-based Molecular orbital methods
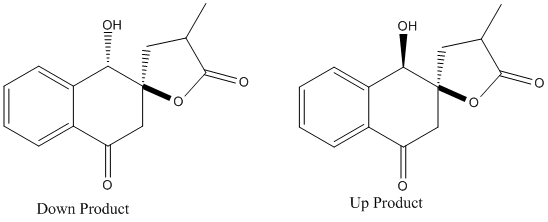
My mini project is on the production of two diastereoisomers of deoxylambertellol[5]. From the supplementory[6] there is a rather indescriptive NMR with a number peaks which appear to show isomerism of the alcohol group, however the information provided says that only the down isomer is produced.
In order to prove my hypothesis that both isomers exist in the product I will be running a number of calculations; 13C NMR and an optical rotation on both species. (Although I have nothing to compare the optical rotation to, it will show the power of computational techniques to help provide information for experimentation)

13C NMR(up); 188.8498000000, 165.9243000000, 146.6933000000, 138.7582000000, 132.4080000000, 131.9202000000, 127.4819000000, 125.8361000000, 124.7793000000, 123.7267000000, 88.3156000000, 73.5580000000, 48.0643000000, 14.2303000000

13C NMR (down); 190.0007000000, 164.9961000000, 147.0164000000, 136.4899000000, 131.6313000000, 131.4919000000, 128.5909000000, 128.3573000000, 126.5425000000, 125.8594000000, 87.0808000000, 74.9348000000, 43.2779000000 & 14.1671000000
While the NMR data given by the journal shows a higher correlation to the "down" isomer, there are definitive peaks which are in the given 13C NMR which are not listed in the data, I believe these peaks correspond to the "up" isomer of the product showing that both isomers exist in the the overall product with the "down" NMR favourable over the up NMR. This may be due to favourable Van der Waals interactions between the alcohol and the 5 membered rings.
| Alcohol pointing down | alcohol pointing up |
|---|---|
| 143.77[9] | 99.13[10] |
References
- ↑ A.G. Shultz, L.Flood, J.P. Springer, J. Org. Chem., 1986, 51. 838: DOI:10.1021/jo00356a016
- ↑ Hyperstable Olefins, Jacs, 1986. 108, p3950-3960DOI:10.1021/ja00398a003
- ↑ DOI:10042/to-5260
- ↑ DOI:10042/to-5259
- ↑ R. Blanc, V. Heran, R Rahmani, L Commeiras and JL Parrain, Org. & Biomol. Chem, 2010 DOI:10.1039/c0ob00448k
- ↑ Supplementory PDF, p8, media:NMR_for_Mini_Project.pdf
- ↑ DOI:10042/to-5257
- ↑ DOI:10042/to-5258
- ↑ DOI:10042/to-5255
- ↑ DOI:10042/to-5256
