Rep:Mod:SHINE11
Experiment 1C - Joseph Shine
Hydrogenation of the Cyclopentadiene Dimer
Comparison of the endo and exo energies
Cyclopentadiene, at room temperature is able to dimerise. It does this through a cycloaddition reaction. The dimer that is formed is purely the , not the .
This is then reduced by hydrogen to give one of the following dihydro derivatives, and . After extensive hydrogenation, the final product is formed, that being the tetrahydro derivative.
A comparison of the total energies of these molecules was required to explain their behaviour. In addition, the different forms of these energies are necessary to explain, precisely, the above processes. The program used for these calculations was Avogadro, with the force field being MMFF94s. When using using this program, a higher energy indicates a greater deviation from ideality, meaning that it is less stable. The main reasons that this method is suited to this type of problem are that, first, it is good at dealing with large molecules.[1] Second, it is more accurate for systems that are close to the models used to reproduce the force field. Third, the main downside to this method, that the number of parameters increases quickly for molecules containing elements apart from C,H,N and O, is not an issue for these molecules. Although Chembio3D is faster and easier to use, Avogadro has the option of breaking down the total energy of the system into its different forms.
| Exo Dimer/kcal/mol | Endo Dimer/kcal/mol | |
|---|---|---|
| Total bond stretching energy | 3.54 | 3.47 |
| Total angle bending energy | 30.8 | 33.2 |
| Total torsion energy | -2.73 | -2.95 |
| Total van der Waals energy | 12.8 | 12.4 |
| Total electrostatic energy | 13.0 | 14.2 |
| Total energy | 55.4 | 58.2 |
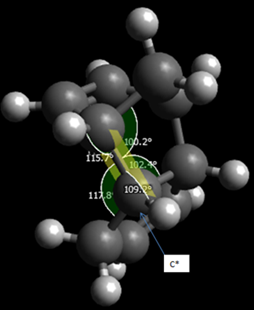
It is clear that the exo dimer is the thermodynamically favoured product. Looking at the distribution of energy, the biggest difference is in the angle bending energy. In this instance, the very large angle bending energy of the endo dimer is due to the fact that the sp2 carbon that becomes sp3 after the dimerisation, has a much greater bond angle than the ideal angle of 109.5o.
The molecule is forced to adjust to this angle to avoid the H-H van der Waals repulsions that would otherwise be present. These are obviously not present in the exo dimer. The endo dimer is the one that is formed, however. This is due to the stabilizing interactions between p orbitals, thereby lowering the energy of the transition state. That is, it is the kinetic product that is formed.
Comparison of the dihydro derivatives
| Dihydro derivative 3/kcal/mol | Dihydro derivative 4/kcal/mol | |
|---|---|---|
| Total bond stretching energy | 3.32 | 2.82 |
| Total angle bending energy | 32.0 | 24.7 |
| Total torsion energy | -1.47 | -0.365 |
| Total van der Waals energy | 13.7 | 10.6 |
| Total electrostatic energy | 5.12 | 5.15 |
| Total energy | 50.6 | 41.3 |
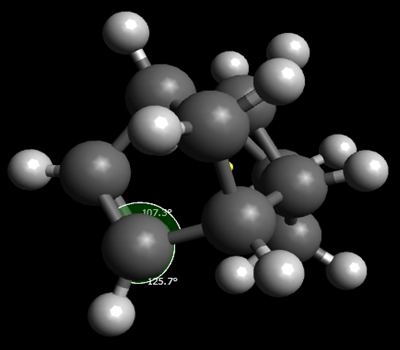
Looking at the total energies of these dihydro derivatives, it shows that dihydro derivative 4 is the thermodynamically favoured product. The key difference between the molecules is caused by the very high angle bending energy of derivative 3. This is, again, to do with the fact that the sp2 carbons are able to gain a bond angle closer to the required one, 120o, in derivative 4. This can be clearly seen from the angle shown below.
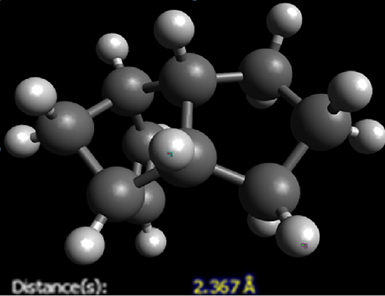
An additional significant difference is in the van der Waals energies. This difference is due to the fact that the protons are closer together in 3, resulting in a more strongly repulsive force. The distance between two protons that gives the most stabilizing interaction is approximately 2.4Ao. Any protons that are closer than this quickly become repulsive. This is demonstrated below.
Atropisomerism demonstrated by an intermediate in the synthesis of Taxol
The two isomers being analysed are this isomer together with the isomer.
| Cis intermediate/kcal/mol | Trans intermediate/kcal/mol | |
|---|---|---|
| Total bond stretching energy | 7.68 | 8.26 |
| Total angle bending energy | 28.3 | 22.1 |
| Total torsion energy | 0.218 | 0.572 |
| Total van der Waals energy | 33.2 | 36.4 |
| Total electrostatic energy | 0.300 | 0.402 |
| Total energy | 70.5 | 74.5 |
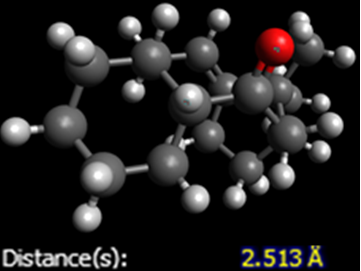
Again, looking at the total energies, it is evident that the cis isomer is the more thermodynamically stable one. Looking at the breakdown of these total energies, the major discriminating factor is the angle bending energies. Interestingly, the trans has the higher angle bending energy. This, again, is to do with ring strain. The C=O, in the trans isomer, is almost in the plane of the ring, meaning that the carbons in the ring are forced into the same plane. Also, there is a reasonable difference in van der Waals energies. The key interaction that causes this is the intramolecular hydrogen bond. This diagram shows that the O-H length is near to the optimum bond length in the cis conformation, but is too long in the trans.
A final point to note is that in the trans isomer, there is overlap between the C-H σ bond and the C-Oπ* bond, shown by the decrease in C-H and C=O, and increase in C-C bond length.
| Cis/Ao | Trans/Ao | |
|---|---|---|
| C=O | 1.24 | 1.238 |
| C-C | 1.539 | 1.546 |
| C-H | 1.105 | 1.1 |
Bredt's rule states that a double bond cannot exist at a bridgehead carbon, in a ring. This does exist in this intermediate, rather, it is hyperstable. It is therefore known as an anti-Bredt molecule. The reasons for this stability are not fully understood. [2]
Spectroscopy of another intermediate in the synthesis of taxol
There are two isomers of an additional intermediate.
The first has the carbonyl cis to the adjacent methyl group.
Taxol Intermediate 17 |
The second, more stable isomer, has the carbonyl trans to the methyl group.
Taxol Intermediate 18 |
Using avogadro to minimise the energy of the system, and then the HPC and gaussview to generate spectra, the following proton NMR was produced. [3],[4],[5],[6]

Comparing to literature.[7]
| Shift/ppm | Degeneracy | Difference between generated and literature shift | |
|---|---|---|---|
| H31 | 5.43 | 1 | 0.22 |
| H49 | 3.24 | 6 | 0.24 |
| H27 | 2.66 | 4 | 0.04 |
| H26 | 2.29 | 6 | 0.09 |
| H25 | 1.96 | 1 | 0.38 |
| H34 | 1.78 | 3 | 0.28 |
| H35 | 1.11 | 3 | 0.01 |
| H51 | 1.06 | 3 | -0.01 |
| H24 | 1.02 | 3 | -.001 |
| Mean | 0.14 |
This is the carbon 13 NMR.
| Shift/ppm | Difference between generated and literature shift | |
|---|---|---|
| C8 | 214 | 3 |
| C4 | 149 | 0 |
| C7 | 117 | 4 |
| C18 | 91 | 16 |
| C14 | 62 | 1 |
| C13 | 59 | 8 |
| C5 | 54 | 3 |
| C2 | 53 | 7 |
| C22 | 49 | 6 |
| C6 | 47 | 6 |
| C21 | 45 | 6 |
| C17 | 42 | 5 |
| C9 | 32 | -3 |
| C15 | 31 | 0 |
| C1 | 31 | 1 |
| C23 | 30 | 4 |
| C3 | 28 | 3 |
| C10 | 27 | 5 |
| C11 | 22 | 1 |
| C16 | 21 | 1 |
| Mean | 4.15 |
Looking at the comparisons for the H spectrum, the mean difference to the literature of 0.14 is not a particularly poor one. The standard deviation of 0.13 and standard error of 0.047 are less respectable.
The carbon 13 table shows a reasonably acceptable mean difference of 4.15. The standard deviation is 3.57 and the standard error is 0.8. These again are not especially bad.
In this instance, using the correction of δcorr = 0.96δcalc + 12.2, was not particularly effective and therefore not used. Another potential correction could be performed for carbons that are bonded to sulphur atoms. These errors, in fact, appear to be random, in that they do not seem to be related to how deshielded the nuclei are. However, almost every simulated peak is more downfield than the literature value, suggesting that the errors may be partly systematic.
Despite these reasonably significant errors, these methods of nmr spectra simulation are, in general, useful tools for analysis of organic compounds. This is due to the fact that C13 shifts tend to be highly sensitive to electronic and steric influences, and yet not sensitive to solvent shifts. They are also spread over a wide spectral range.[8]
Analysis of the synthesised epoxides
There were two epoxides that were synthesised in experiment 1S. The first one was trans-stillbene epoxide and . The second was trans-β-methyl styrene epoxide and .
Catalysts
These were made using Jacobsen's catalyst and Shi's catalyst.[9] [10] [11] [12] [13]
Analysing the crystal structure of the using conquest and mercury yielded an interesting result, namely that the hydrogens on the tertiary butyl groups are very close together. The shortest distance found between them was 2.421Ao, indicating a stabilizing interaction. Another interesting result is that these protons also have a distance of 2.779Ao-2.458Ao between themselves and the oxygens, again showing an attractive interaction.
Looking at the crystal structure of the , the key point to note is that the bond lengths of the C-O, where the carbon is an anomeric centre, are different. This is a direct result of the anomeric effect. A similar version of this form of orbital overlap is discussed above. The bond that is closer to the δ+ carbonyl carbon is the longer one. This is to do with the fact the δ+ C withdraws electron density away from the O lone pairs, meaning that less can donate into the antibonding orbital. The C-O therefore has less electron density in it and the bond is not shortened as much. [14]
Optical rotation
These are all [alpha] type rotations, the wavelength used is 589nm and the solvent is chloroform.
| Concentration/g/100ml | Optical rotationo | |
|---|---|---|
| (R,R) TBMS | 46.77 | |
| Lit (R,R) TBMS | 0.63 | 45.9 |
| (R,R) Trans stillbene | 297.82 | |
| Lit (R,R) trans stillbene | 0.85 | 250.8 |
These values are reasonably accurate. In general, an [alpha] value lower than 100o can lead to increasingly unreliable results. This is clearly not the case here however.
Enantiomeric excess
| ΔG | k | ee% | |
|---|---|---|---|
| State 1 | 0.0044 | 102 | 99.02 |
| State 2 | 0.0022 | 9.89 | 89.88 |
| State 3 | 0.0062 | 726 | 99.86 |
| State 4 | 0.0080 | 5010 | 99.98 |
The gibbs free energies that were calculated were converted to J/mol-1 from Hartrees.
Non covalent interactions
This is the first transition state of the (R,R) Shi epoxidation of trans stillbene. It was simulated using Gaussian.[15] The orange ring indicates a bond forming and is therefore ignored. The green indicates a slightly attractive interaction.
Orbital |
There is clearly an attractive interaction between the aromatic rings on trans stillbene and the protons on the Shi catalyst. The catalyst can only do this on one side of the trans stillbene, since it will otherwise gain a very large amount of angle bending energy.
Alternatives
One interesting alternative for epoxidation is . It is a natural product with an optical rotation of 26o at 3.1g/100ml in chloroform.
Conclusion
The computational methods discussed are very powerful and fast tools for analysis of simple C,H,N and O containing organic molecules. The errors begin to increase rapidly, however, when heavier atoms are introduced. The programs that can be used on such molecules, such as MAGIC, tend to be much slower. The main difficulty is the high angular momentum of the occupied orbitals, particularly in the actinides. [16]
References
- ↑ A. K. Rappe , C. J. Casewit , K. S. Colwell , W. A. Goddard III, W. M. SkiffJ. Am. Chem. Soc., 1992, 114, 10024–10035. ,C. J. Casewit , K. S. Colwell , A. K. Rappe J. Am. Chem. Soc., 1992, 114, 10046–10053 ,C. J. Casewit , K. S. Colwell , A. K. Rappe J. Am. Chem. Soc., 1992, 114, 10035–10046
- ↑ 488 Bull,Korean Chem., 1997, 18.
- ↑ K. Mori, "Synthetic examination of incorrectly proposed structures of biomolecules", The Chemical Record, 2005, ii5, 1-16.
- ↑ K L. McPhail et al, "Survey of marine natural product structure revisions: A synergy of spectroscopy and chemical synthesis", Bioorganic & Medicinal Chemistry, 2011, 19, 6675–6701.(there are some serious errors in the correctedstructures here for obtusallene, viz, Structural Reassignment of Obtusallenes V, VI, and VII by GIAO-Based Density Functional Prediction)
- ↑ M. G. Banwell et al, "Structure of the Lycorinine Alkaloid Nobilisitine A", J. Org. Chem., 2011, 76, 8560–8563
- ↑ M. W. Lodewyk , C. Soldi , P. B. Jones, M. M. Olmstead , J. Rita , J. T. Shaw, and D. J. Tantillo, "The Correct Structure of Aquatolide—Experimental Validation of a Theoretically-Predicted Structural Revision", J. Am. Chem. Soc., 2012, 134, 18550–18553
- ↑ L.A.Paquette, N.A.Pegg, D.Toops, G.D.Maynard, R.D.Rogers, J. Am. Chem. Soc., 1990, 112, "277-283".
- ↑ Organic Letters, 2006, 8, "2895-2898".
- ↑ A. Burke , P. Dillon , Kyle Martin and T. W. Hanks,"Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", J. Chem. Educ., 2000, 77, 271
- ↑ O. A. Wong , B. Wang , M-X Zhao and Y. Shi J. Org. Chem., 2009, 74, 335–6338
- ↑ J. Hanson,J. Chem. Educ., 2001, 78, 1266
- ↑ E. N. Jacobsen , W. Zhang , A. R. Muci ,J. R. Ecker , L. Deng J. Am. Chem. Soc., 1991, 113, 7063–7064
- ↑ M. Palucki , N. S. Finney , P. J. Pospisil , M. L. Güler , T. Ishida , and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 948–954
- ↑ Zhi-Xian Wang, Yong Tu, Michael Frohn, Jian-Rong Zhang, and Yian ShiJ. Am. Chem. Soc., 1997, 119, 11224-11235
- ↑ J. L. Arbour, H. S. Rzepa, J. Contreras-García, L. A. Adrio, E. M. Barreiro, K. K. Hii, Chem.Euro. J., 2012, 18, 11317–11324
- ↑ Willetts, A., Gagliardi, L., Ioannou, A.G., Simper, A.M., Skylaris, C.-K., Spencer, S. and Handy, MAGIC: an integrated computational environment for the modelling of heavy-atom chemistryInternational Reviews in Physical Chemistry., 2000, 19, 327-362.