Rep:Mod:SE7EN3NS
Optimising BH3 molecule
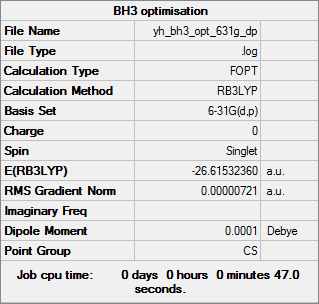
BH3 molecule optimised using 6-31G(d,p) Gaussview calculation
The link to the log file of optimised BH3 molecule using 6-31g(d,p) calculation: YH BH3 OPT 631G DP.LOG
The energy of optimised BH3 molecule using 6-31G(d,p) Gaussview calculation is -26.61532 a.u. to 5 decimal places.
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000064 0.001800 YES
RMS Displacement 0.000039 0.001200 YES
Predicted change in Energy=-1.126784D-09
Optimization completed.
-- Stationary point found.
All items are converged and the RMS gradient norm is close to 0, meaning that the molecule was optimised properly.
Optimised BH3 using 6-31G(d,p) |
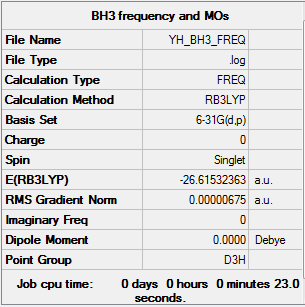
BH3 molecule with D3h symmetry frequency and MOs calculation
The link to the frequency calculation log file: YH BH3 FREQ.LOG
The BH3 molecule that was optimised using 6-31G(d,p) Gaussview calculation had Cs point group regardless. Thus, the molecule was symmeterised into D3h point group which was then optimised again using the same calculation method.
Smf115 (talk) 01:18, 17 May 2018 (BST)Nice aknowledgement of the symmetrisation done.
The energy is -26.61532 a.u. up to 5 decimal places.
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000053 0.001800 YES RMS Displacement 0.000027 0.001200 YES Predicted change in Energy=-1.076094D-09 Optimization completed. -- Stationary point found.
Once again, all items are converged and the RMS gradient norm is close to 0 meaning that the symmeterised BH3 molecule was optimised properly.
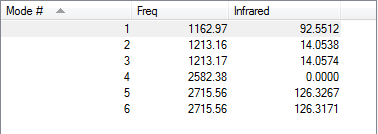
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
1 2 3
A2" E' E'
Frequencies -- 1162.9677 1213.1634 1213.1661
D3h symmetry BH3 |
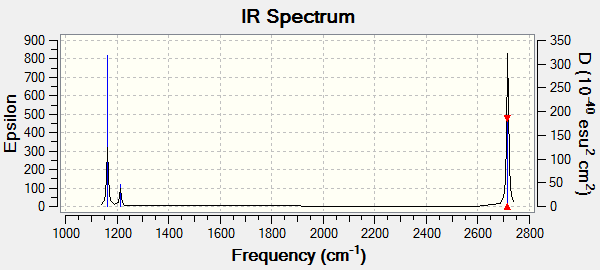
The IR spectra of optimised BH3 molecule:
Although the frequency table shows 6 different vibrational mode, only 3 peaks are observed in the IR spectrum. There are two sets of degenerate vibrational modes and one mode with an extremely low intensity which leads to having only 3 peaks visible in the IR spectra.
The MO diagram of optimised BH3:
The LCAOs are quite accurate in representing the real MOs!
Association energies: Ammonia-Borane
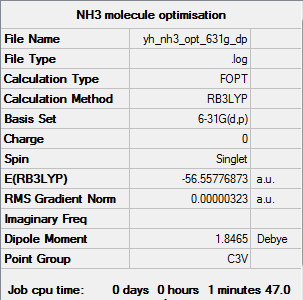
NH3 molecule optimised using 6-31G(d,p) Gaussview calculation
The link to the log file of optimised NH3 molecule using 6-31g(d,p) calculation: YH NH3 OPT 631G DP.LOG
The energy of optimised NH3 molecule using 6-31G(d,p) Gaussview calculation is -56.55777 a.u. to 5 decimal places.
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES Predicted change in Energy=-9.844491D-11 Optimization completed. -- Stationary point found.
All items are converged and the RMS gradient norm is close to 0 meaning that the molecule was optimised properly.
Optimised NH3 using 6-31G(d,p) |
The optimisation of NH3 molecule gave the correct point group, C3v, and thus, it didn't have to be symmeterised and then re-optimised.
NH3 molecule frequency calculation
The link to the log file of optimised NH3 molecule frequency calculation: YH NH3 FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
1 2 3
A E E
Frequencies -- 1089.7603 1694.1865 1694.1865
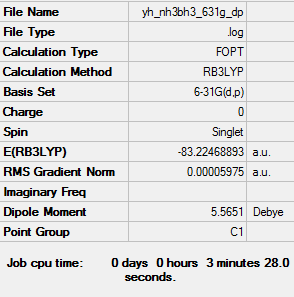
Ammonia borane molecule optimised using 6-31G(d,p) Gaussview calculation
The link to the log file of optimised ammonia borane molecule using 6-31g(d,p) calculation: YH NH3BH3 631G DP.LOG
The energy is -83.22469 a.u. up to 5 decimal places.
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000513 0.001800 YES RMS Displacement 0.000296 0.001200 YES Predicted change in Energy=-1.631175D-07 Optimization completed. -- Stationary point found.
All items converged meaning that the molecule was optimised properly
Optimised NH3BH3 using 6-31G(d,p) |
The link to the NH3BH3 frequency calculation log file: YH NH3BH3 FREQ.LOG
Low frequencies --- -0.0016 -0.0010 -0.0001 18.4381 27.1658 40.5145 Low frequencies --- 266.4358 632.3947 639.7877
1 2 3
A A A
Frequencies -- 266.4278 632.3946 639.7875
Determination of the dissociation energy of NH3BH3
E(NH3) = -56.55777 a.u.
E(BH3) = -26.61532 a.u.
E(NH3BH3) = -83.22469 a.u.
ΔE = E(NH3BH3) - [E(NH3) + E(BH3)] = -0.05160 a.u. which is equivalent to -135.48 kJ/mol
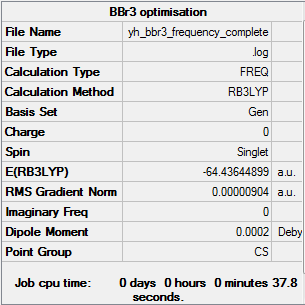
Optimising BBr3 molecule
The energy is -64.43644 a.u. to 5 decimal places
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000092 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-1.575225D-09 Optimization completed. -- Stationary point found.
All items are converged meaning that the molecule was optimised properly.
Low frequencies --- -5.0430 -0.0002 -0.0001 0.0001 2.0030 3.3783 Low frequencies --- 155.8909 155.9427 267.7109
1 2 3
A' A' A'
Frequencies -- 155.8909 155.9427 267.7109
Optimised BBr3 using 6-31G(d,p) GEN |
Project: Investigating Aromaticity, Benzene vs Borazine
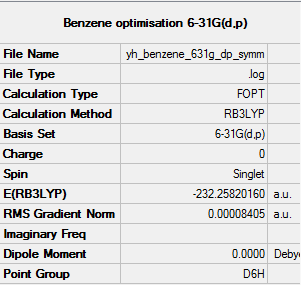
Benzene optimised using 6-31G(d,p) Gaussview calculation
The link to the log file of optimised Benzene molecule using 6-31g(d,p) calculation: YH BENZENE 631G DP SYMM.LOG
The energy is -232.25820 a.u. to 5 decimal places
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000077 0.000300 YES Maximum Displacement 0.000824 0.001800 YES RMS Displacement 0.000289 0.001200 YES Predicted change in Energy=-4.246203D-07 Optimization completed. -- Stationary point found.
All items are converged meaning that the molecule was optimised properly.
Optimised Benzene using 6-31G(d,p) |
The link to benzene frequency calculation log file: YH BENZENE FREQ.LOG
Low frequencies --- -2.1456 -2.1456 -0.0089 -0.0042 -0.0042 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
1 2 3
E2U E2U E2G
Frequencies -- 413.9768 413.9768 621.1390
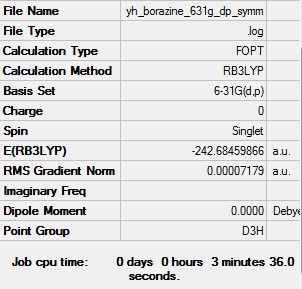
Borazine optimised using 6-31G(d,p) Gaussview calculation
The link to the log file of optimised Borazine molecule using 6-31g(d,p) calculation: YH BORAZINE 631G DP SYMM.LOG
The energy is -242.68469 a.u. to 5 decimal places.
Item Value Threshold Converged? Maximum Force 0.000077 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000273 0.001800 YES RMS Displacement 0.000103 0.001200 YES Predicted change in Energy=-1.107323D-07 Optimization completed. -- Stationary point found.
All items are converged meaning that the molecule was optimised properly.
Optimised Borazine using 6-31G(d,p) |
The linke to borazine frequency calculation log file: YH BORAZINE FREQ.LOG
Low frequencies --- -10.4805 -0.7848 -0.0007 -0.0005 0.0009 10.9921 Low frequencies --- 288.4643 290.4506 403.9219
1 2 3
A2 B1 B1
Frequencies -- 288.4640 290.4503 403.9214
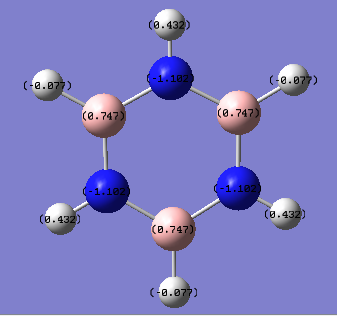
Comparing charge distribution of benzene and borazine
Charge distribution of benzene:
Charge distribution of borazine:
| element | charge |
|---|---|
| C | -0.239 |
| H | 0.239 |
| element | charge |
|---|---|
| B | 0.747 |
| N | -1.102 |
| H, attached to B | -0.77 |
| H, attached to N | 0.432 |
The charge is very unevenly distributed in a borazine molecule. The cyclo-arrangement of boron and electronegative nitrogen resulted in the charge being concentrated on the nitrogen atoms. In benzene, the 6 carbons in planar configuration equally contributes 1 electron to to aromaticity to fulfill Huckel's law. In the borazine, however, the 3 nitrogens each contribute 2 electrons with each borons having an empty p orbital.
Smf115 (talk) 23:16, 15 May 2018 (BST)Clear labelling of the charges in the table. However, the analysis doesn't consider the key points such as the atoms electronegativities and the symmetry of the molecules.
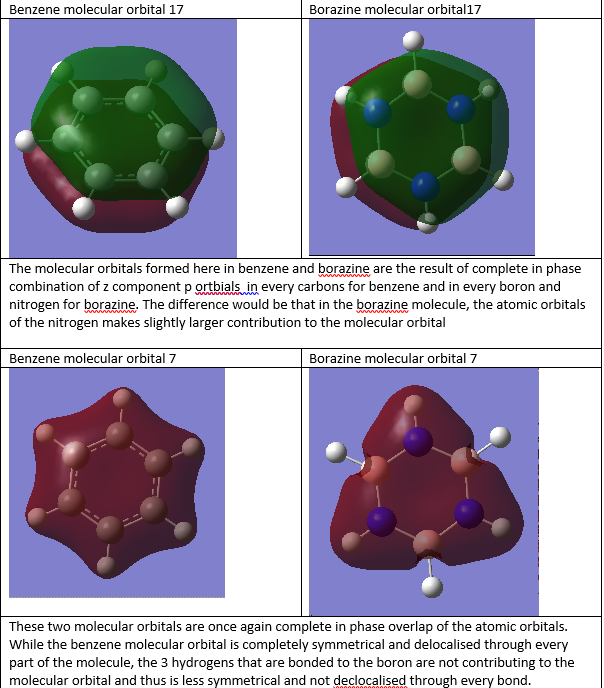
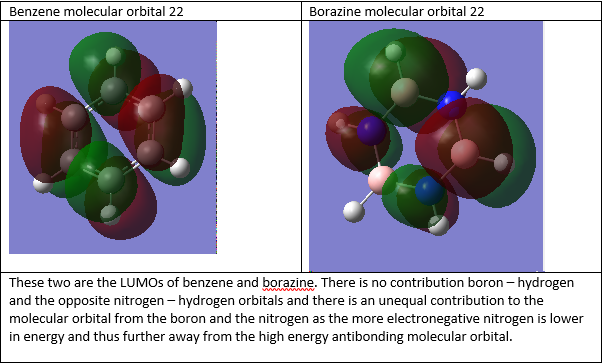
Smf115 (talk) 01:21, 17 May 2018 (BST)Good attempt at the MO comparison. The pi- or sigma- nature of the orbitals, the charcters and the symmetries could be considered to improve the discussion and a wider range of MOs would have been nice.
The sigma orbitals can also contribute to delocalisation of electrons not just the pi orbitals that form from overlap of pz orbitals. Thus one can not determine the aromaticity of a certain molecule just by looking at the overlap of pz atomic orbitals as there might be overlap of sigma orbitals contributing to the delocalised system.
Aromaticity in the most basic term is the delocalisation of pi electron system resulting in electron density above and below the plane of the molecule and this provides extra stabilisation. The aromaticity results certain bond length being in between that of single and a double bond. A ring compound that has 4N+2 number of pi electrons where n is a positive integer, the compound is aromatic and this is called the Huckel's law. Molecules that exhibit aromaticity generally undergo reaction that maintains the delocalised pi system.
Smf115 (talk) 01:24, 17 May 2018 (BST)Overall a good report. More key terminology and development of ideas in the project section would help improve the discussions.