Rep:Mod:SAS123
Ammonia
| Molecule | NH3 |
| Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Energy | -56.55776873 au |
| RMS Gradient | 0.00000485 au |
| Point Group | C3v |
| N-H Bond Length | 1.01798 Å |
| H-N-H Bond Angle | 105.741° |
Items Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 |
Vibrations
Animating vibrations
1. How many modes do you expect from the 3N-6 rule?
I would expect 6 vibrational modes as there are 4 atoms and (3x4)-6=6.
2. Which modes are degenerate (ie have the same energy)?
Vibrational modes 2 and 3, and 5 and 6 are degenerate as they have the same energy.
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Vibrational modes 1,2 and 3 are bending modes and modes 4,5 and 6 are stretching modes.
4. Which mode is highly symmetric?
Vibrational mode 4 is the highly symmetric stretch.
5. One mode is known as the "umbrella" mode, which one is this?
Mode 1 is the "umbrella" mode
6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
I would expect 4 bands in an experimental spectrum of gaseous ammonia, as there are 4 non-degenerate modes.
Atomic Charge
I would expect the charge on the N atom to be more negative than the H atom, as the N atom is more electronegative and draws electron density from its bonds and has a lone pair of electrons, making it more negatively charged. the charge on the N atom is -1.125 and the charge on the H atoms is 0.375.
Haber-Bosch Process
Nitrogen
| Molecule | N2 |
| Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Energy | -109.52412868 au |
| RMS Gradient | 0.00000060 au |
| Point Group | D∞H |
Items Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 |
Hydrogen
| Molecule | H2 |
| Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Energy | -1.17853936 au |
| RMS Gradient | 0.00000017 au |
| Point Group | D∞H |
Items Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 |
Haber-Bosch Reaction Energy Calculation
E(NH3)= -56.55776873 au
2*E(NH3)= -113.11553746 au
E(N2)= -109.52412868 au
E(H2)= -1.17853936 au
3*E(H2)= -3.53561808 au
ΔE= -0.0557907 au = -146.48 kJ/mol
Therefore we can see that the product of ammonia is thermodynamically more stable than the reactants of hydrogen and nitrogen. This value was compared to the literature value of -92 kJ/mol[1], which was smaller in magnitude than the value calculated above. This literature value is an enthalpy change and in the calculation there were no specific details of the pressure or volume, as it is purely the change in energy and stability going from hydrogen and nitrogen to ammonia.
Chlorine
| Molecule | Cl2 |
| Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Energy | -920.34987886 au |
| RMS Gradient | 0.00002510 au |
| Point Group | D∞H |
Items Table
Item Value Threshold Converged? Maximum Force 0.000043 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000121 0.001800 YES RMS Displacement 0.000172 0.001200 YES
Cl2 |
Vibrations
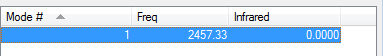
There is only one vibrational frequency, as was expected as Chlorine is a linear molecule and so the expression 3N-5 is used. As chlorine has 2 atoms, the number of frequencies is (3x2)-5=1. This is a low vibrational frequency as the chlorine atoms are large and heavy, giving a large reduced mass and therefore short wavenumber and frequency. However, as chlorine is a diatomic molecule, the vibrational mode is IR inactive as there is no change in dipole moment. As there would be no IR spectrum formed by Chlorine, there was no literature IR spectrum to compare to, as the only vibrational mode was IR inactive.
Charge
As chlorine is a diatomic molecule, the bond is not polar and so there is no net charge on either atom.
Molecular Orbitals
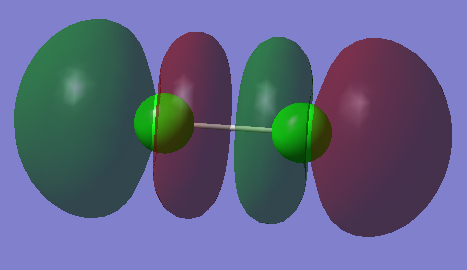
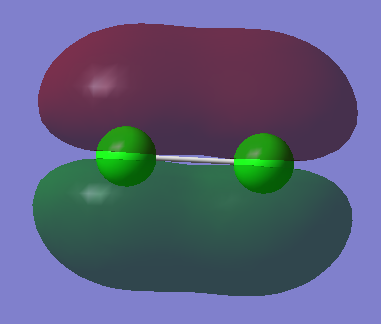
This orbital is the LUMO of the molecule, it is a σ* orbital and has a symmetry label of ungerade. It has an energy of -0.14206 au and is created by the interaction of the 3pz AOs out of phase from the chlorine atoms. It is unoccupied and is an antibonding orbital.
This orbital is the HOMO of the molecule, it is a π* orbital and has a symmetry label of gerade. It has an energy of -0.31361 au and is created by the interaction of the 3px AOs out of phase from the chlorine atoms. It is occupied and is an antibonding orbital.
This orbital is in the HOMO/LUMO region, it is a π orbital and has a symmetry label of ungerade. It has an energy of -0.40695 au and is created by the interaction of the 3px AOs in phase from the chlorine atoms. It is occupied and is a bonding orbital.
This orbital is in the HOMO/LUMO region, it is a σ orbital and has a symmetry label of gerade. It has an energy of -0.47392 au and is created by the interaction of the 3pz AOs in phase from the chlorine atoms. It is occupied and is a bonding orbital.
This orbital is slightly deeper in energy than the HOMO/LUMO region, as chlorine is an electronegative element, the MO is much deeper in energy. It is a σ* orbital and has a symmetry label of ungerade. It has an energy of -0.77746 au and is created by the interaction of the 3s AOs out of phase from the chlorine atoms. It is occupied and is an antibonding orbital.
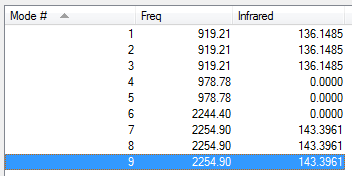
Silane Vibrations
We can see that there are 3N-6 vibrational modes as the molecule is non-linear, so in this case, there should be (3x5)-6= 9 vibrational modes. However some modes are degenerate and so there are only 4 different non-degenerate vibrational modes.
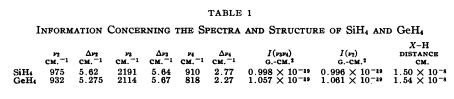
Below is a literature table of vibrations of silane. [2]
However we can see from this, that because the two non-degenerate modes at around 2250 cm-1 overlap and so only one peak is observed in the spectrum, as demonstrated in the table.
- ↑ This is the literature value of the enthalpy change of the Haber-Bosch process http://www.chemguide.co.uk/physical/equilibria/haber.html
- ↑ Table of literature IR vibrations of Silane https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1078306/pdf/pnas01627-0016.pdf