Rep:Mod:SAITYM945
Year 3 Physical Computational
Cope Rearrangement
Introduction
Cope Rearrangement is a [3,3]-sigmatropic rearrangement of 1,5-dienes. Cope rearrangement of 1,5-hexadiene had been subject to many computational studies and it is now generally accepted that the [3,3]-sigmatropic shift mechanism occurs via a 'boat' or a 'chair' transition. The objective of this tutorial course is to work out the low energy minima and the transition structure on the C6H10 potential energy surface to determine the preferred reaction mechanism. All conformers are labeled as the following from Appendix 1

Optimizing the Reactants and Products
1,5-hexadiene (anti)
In the first part of this exercise, the 1,5-hexadiene with an 'anti-linkage'(the 4 center carbon atoms are of app conformation)was drawn on Gaussian, and optimized using the HF/3-21G level of theory.

The optimized 'anti' 1,5-hexadiene had total energy of -231.69260235 Hartrees with point group of C2 (anti 1).
1,5-hexadiene (gauche)
A 1,5-hexadiene molecule with 'gauche-linkage' was drawn and similarly to the example above was optimized using the HF/3-21G level of theory. The optimized 'gauche' 1,5-hexadiene molecule (gauche 4) had electronic energy of -231.69153035 Hartrees and a point group of C2 .

I had predicted that the gauche conformer is higher in energy than the anti conformer and this is due to higher stabilization energies in 'anti' form than the 'gauche' form. The anti form have 4 σC-H/σ*C-H and 2σC-C/σ*C-C interactions which have higher stabilizing energies than the 2 σC-H/σ*C-H , 2 σC-C/σ*C-H and 2 σC-H/σ*C-C interactions present in the gauche form. The anti form also avoids the increased steric repulsion from the carbon atoms in the gauche conformation which also lower the energy of the anti conformer relatively. This prediction agrees with the energy results when you compare the total energies between 'anti 1' and 'gauche 4' where 'anti 1' conformer has lower energy. However out of the 10 conformers with distinct energy levels, when optimized using the HF/3-21G level of theory, gauche 3 is the lowest energy conformer. A possible explanation could be due to favorable orbital interaction between two π orbitals on the terminal carbons overcompensating for the steric repulsion and lower stablisation energies in gauche 3 conformer allowing it to lie in a lower energy level than the anti conformers.[1]

Gauche 2 had electronic energy level of -231.69166701 Hartrees with a point group of C2.
Optimization of Ci anti 2 conformer of 1,5-hexadiene
The 'anti 2' conformer which is the lowest energy 'anti' conformer was drawn and optimized at the HF/3-21G level of theory giving electronic energies of -231.69253528 Hartrees and point group of Ci. The HF/3-21G optimized 'anti 2' 1,5-hexadiene was then re-optimized at the B3LYP/6-31G* level giving electronic energy at -234.61170280 Hartrees. The structures of both anti 2 conformer optimized at HF/3-21G and B3LYP/6-31G* were compared and the energies as well as the dihedral angles were seen to be significantly different. The conformer optimized at the HF/3-21G level had dihedral angles of 114.66879o where as the conformer optimized at the B3LYP/6-31G* level had a dihedral angle of 118.52813o. This change in dihedral angle is followed by a lowering of energy in the molecule. This could be a consequence of the dihedral angle, when re-optimized, moving closer to 120owhich is the favored dihedral angle for atoms on the carbon adjacent to the carbon with the double bond. This 1200angle allows two σ bonds to interact with the π system rather than one at 90o as well as eclipsing one of the hydrogen atom to the cis hydrogen atoms where the interaction between those hydrogen atoms is slightly van der waals attractive further lowering the energy. This favored conformation is called the A1,3 eclipsed conformation and explains the shift of the dihedral angle going closer to 120o when re-optimized..
Frequency calculation were done on the re-optimized 'anti 2' 1,5-hexadiene and the calculated energy level were noted down in Table 1.


| Different energies with correction | Energy/Hatrees |
|---|---|
| Sum of electronic and zero-point Energies | -234.469212 |
| Sum of electronic and thermal Energies | -234.461856 |
| Sum of electronic and thermal Enthalpies | -234.460912 |
| Sum of electronic and thermal Free Energies | -234.500821 |
Optimizing the Boat and Chair transition state
Optimizing the Chair transition
An allyl fragment (CH2CHCH2)was drawn and subsequently optimized at the HF/3-21G level. This optimized fragment was then copied and pasted, and orientated in such a way that it formed a 'guess' transition structure for the chair transition. The terminal carbons were modeled in the guess transition so that it was roughly 2.2A apart. This transition state was then optimized using two different methods, one method by computing the force constant at the beginning of the calculation and the other by using the redundant coordinate editor.
Computing the force constant and optimizing to a TS(Berny)
Using the HF/3-21G level of theory, the guess transition state was optimized to a TS(Berny) with Force constant calculated once. The optimized structure had only one imaginary vibration at -817.9cm-1,bond length between terminal carbons at 2.02 and electronic energy at -231.61932245 Hartrees


Optimizing the transition using a redundant coordinate editor
The bond length or the distance between the two terminal carbons opposite to each other in the guess transition was frozen and fixed using the redundant coordinate editor. The guess transition was then optimized to a minimum at the HF/3-21G level initially. When the optimization completed, the bonds were then relaxed again using the redundant coordinate editor and optimized this time to a TS(Berny) without calculating the force constant. The optimized chair transition gave only one imaginary frequency at -817.85-1, bond length between terminal carbons at 2.02 and electronic energy at -231.61932243 Hartrees
Optimizing the Boat transition using the QST2 method
The QST2 method was used to optimize the boat transition at the HF/3-21G level. This method requires you to input the reactants and the products, and automatically calculates the transition state. It is important to number the atoms of the reactants and the product accordingly and the transition state structure cannot be too far away from the structure of the reactants and the product or the method will not work. This was outlined when trying to optimize the boat transition using 'anti 2' 1,5-hexadiene modeled as the reactant and the product where it failed to rotate around the center bond and processed the wrong transition state. It is however still possible to optimize the boat transition starting from 'anti 2' by manually modelling closer to the gauche form and processing it under the QST2 method. The QST2 method successfully optimized the boat transition giving an imaginary frequency at -839.87cm-1, bond length at 2.14A and electronic energy at -231.60280240 Hartrees.
 |
 |
 |
 |
 |
Using the Intrinsic Reaction Coordinate method (IRC)
The IRC method allows you to follow the lowest energy path from the transition to the local minimum on a potential energy surface. The IRC method was applied to the chair transition and it can be seen that the chair transition connects to form the gauche 2 conformer.
 |
 |
Re-optimization of transition states using the B3LYP/6-31G* level
The chair and boat transitions were re-optimized in the B3LYP/6-31G* level. Both transitions lowered its energy significantly. The chair transition gave electronic energy of -234.55698303 Hartrees, bond length of 1.96A and imaginary frequency at -565.54cm-1. The boat transition gave electronic energy of -234.54309308 Hartrees, bond length of 2.20Å and imaginary frequency at -530.46cm-1. Table 2 shows the energies of the transitions optimized at the HF/3-21G and B3LYP/6-31G* level. Gauche 3 is the lowest energy conformer relatively and was therefore used as the reactants for these transitions to work out the activation energies subsequently. Activation energies are in kcal/mol where 1 hartree = 627.509 kcal/mol.
Comparison of Energies/hartree (Table 2)
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| Chair TS | -231.619322 | -231.466698 | -231.461340 | -234.556983 | -234.414929 | -234.409009 |
| Boat TS | -231.602802 | -231.450929 | -231.445300 | -234.543093 | -234.402341 | -234.396006 |
| gauche 3 (Reactant) | -231.692661 | -231.539486 | -231.532646 | -234.611329 | -234.468693 | -234.461464 |
Activation energies of the transition states in kcal/mol (Table 3)
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Experimental[2][3] | |
|---|---|---|---|---|---|
| 0K | 298.5K | 0K | 298.5K | 0K | |
| ΔE of Chair TS | 45.675125 | 44.745156 | 33.737393 | 32.915984 | 33.5 ± 0.5 |
| ΔE of Boat TS | 55.570314 | 54.810401 | 41.636477 | 41.075484 | 44.7 ± 2.0 |
The activation energies are generally in good agreement to experimental values suggesting computational method are a good method in estimating activation energies of reactions.
As it can be seen in the table and the models, when re-optimized at the B3LYP/6-31G* level, although the geometry of the transition states do not change too much, the energy difference are very large. The differences between the HF/3-21G and B3LYP/6-31G* optimized structures are around 2.9 hartree which is around 1820kcal/mol. This is a massive energy difference and empathizes the importance of optimizing from lower level to higher, in this case HF/3-21G to B3LYP/6-31G* level of theory.
The Diels Alder Cycloaddition
Cycloaddition of Ethylene and Butadiene
Both ethene and cis-butadiene were optimized using the AM1 semi-empirical molecular orbital method, and then at the HF/3-21G and B3LYP/6-31G* level of theory. The energies are given in Table 5 where combined energies will be used to work out the activation energies of the transition state. The HOMO and the LUMO of cis-butadiene are anti-symmetric and symmetric respectively and the HOMO and the LUMO of ethene are symmetric and anti-symmetric respectively.
Energies of ethene and cis-butadiene at AM1 semi-empirical and B3LYP/6-31G* level (Hartree) (Table 5)
| AM1 semi-empirical | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| cis-butadiene | 0.048797 | 0.134552 | 0.138573 | -155.985949 | -155.900825 | -155.896783 |
| ethene | 0.026190 | 0.077198 | 0.080252 | -78.587458 | -78.536231 | -78.533189 |
| Combined energies | 0.074987 | 0.21175 | 0.218825 | -234.573407 | -234.437056 | -234.429972 |
| HOMO(a) | LUMO(s) | HOMO (s) | LUMO (a) |
|---|---|---|---|
 |
 |
 |
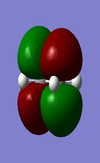 |
Ethylene+cis butadiene transition structure
The transition structure was characterized and optimized using the QST2 method. Cyclohexene was optimized at the AM1 semi-empirical molecular orbital method, and then at the HF/3-21G and B3LYP/6-31G* level of theory to be inputted as the product for the QST2 method, with ethene and cis-butadiene as the reactants. The QST2 method gave a Ethylene+ cis butadiene transition structure, where when IRC was run on it, connected to give the cyclohexene, confirming that it is in fact the wanted Diels-Alder transition state. The transition state was re-optimized at the B3LYP/6-31G* level.
| reactants | product | transtion state | IRC | Vibration at imaginary freqeuncy | |
|---|---|---|---|---|---|
 |
 |
 |
 |
 |
| HOMO of transition state (a) | LUMO of transition state (s) | lowest positive frequency vibrations |
|---|---|---|
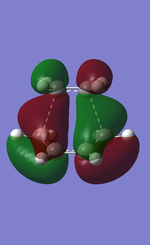 |
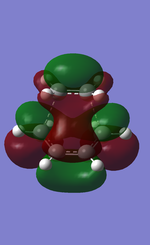 |
 |
The molecular orbitals of the transition state is plotted above with HOMO anti-symmetric and LUMO symmetric with respect to the plane. The bond length of the partially formed C-C bond was 2.27Å. Typical sp3 and sp2 carbon carbon bond length are 1.54Å and 1.35Å respectively. The van der Waals radius of the C atom is 1.70Å. The partial bond length of 2.27Å is much bigger than the typical C-C bond lengths and therefore is a very weak bond. However it is smaller than the sum of the Van der Waals radius for carbon and therefore there must be an attractive force between the carbons. This partial bond can therefore be concluded as a result of Van der Waals attractive force between the carbon atoms.
The transition state gave a imaginary frequency of -524.78cm-1 and looking at the vibration in the model, it can be clearly seen that the two bond formed are formed synchronously. The lowest positive frequency vibration at 135.76cm-1 which can be seen above, is an asynchronous movement along the horizontal plane between the molecules.
The calculated HOMO of the transition is anti-symmetric MO and is an interaction between the LUMO of ethene and the HOMO of the cis-butadiene and is a bonding MO between the carbons of ethene and cis-butadiene. This reaction is allowed due to the correct orientation of the MO allowing good overlap when the two molecules approach.
| AM1 semi-imperical | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| electronic energies | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | electronic energies | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| transition state | 0.11165469 | 0.253276 | 0.259454 | -234.54389648 | -234.403321 |
-234.396903 |
| activation energies(kcal/mol) | 26.06 | 25.50 | 21.17 | 20.75 | ||
| Experimental activation energies(kcal/mol)[4] | 25.1 (0K) | 32.8-34.3(800K) |
The activation energy of AM1 semi-empirical level is generally in good agreement with experimental values. This suggest that computational methods in predicting energies of diels alder reaction at the AM1 semi-empirical level is a valid method
Diels-Alder reaction between Cyclohexa-1,3-diene and maleic anhydride
Both the endo- and the exo- transition structure were characterized and optimized using the QST2 method at the AM1 semi-empirical level and then at HF/3-21G and B3LYP/6-31G* level. Exo form gave partial bond length at 2.29Å and imaginary frequency at -448.55cm-1. The endo form gave partial bond length at 2.268Å and imaginary frequency at -447.04cm-1 . The energies of the transitons are listed in a table below. There is a notable difference in the partial bond length in the endo and the exo form with endo being slightly shorter. This suggest that the partial bond length is stronger in the endo transition and is more likely to be favored.
| endo- | HOMO of endo | imaginary frequency vibration | exo- | HOMO of exo | imaginary frequency vibration |
|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
| AM1 semi-imperical | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| electronic energies | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | electronic energies | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| endo | -0.05150479 | 0.133494 | 0.143684 | -612.68339669 | -612.502141 | -612.491787 |
| exo | -0.05041967 | 0.134883 | 0.144882 | -612.67931093 | -612.498012 | -612.487661 |
| heading | relative electronic energies (kcal/mol) | |
|---|---|---|
| AM1 | B3LYP/6-31G* | |
| endo | 0 | 0 |
| exo | 0.680 | 2.59 |
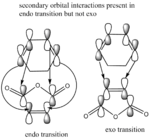
Table above shows that the endo transition is lower in energy than the exo transition with agreement at two level of theory. Endo product predominates due to it being the kinetic product with lower energy transition state. This is due to the secondary orbital overlap illustrated in a diagram on the right. The HOMO of both transitions consist of interactions between the LUMO of the malaeic anhydride and HOMO of the 1,3-cyclohexadiene which are both anti-symmetrric with respect to plane and therefore can overlap and react. However in the endo transition state, there is an extra orbital interaction that is not present in the exo transition thus leading to lower endo transitions energies. This reaction therefore is under kinetic control.Although secondary orbital overlap is likely to be the predominate force for endo being favored, another factor maybe that the exo transition state is stericly more hindered the endo transition state. This is due to the closer positioning of the sp3 hydrogen atom to the C=O carbon atom present in exo-TS but not endo-TS. The distance between these atoms is 2.53Å which is smaller than the combined van der waals radius of hydrogen and carbon atoms and therefore repulsive. [5][6]
reference
- ↑ Rocque, B. G., Gonzales, J. M., & Schaefer, H. F. (2002). An analysis of the conformers of 1,5-hexadiene. Molecular Physics, 100(4), 441–446. doi:10.1080/00268970110081412
- ↑ Imperial College Chemwiki
- ↑ James Edward Kabrhel, August 2006,Part I. Is a Cope Rearrangement Viable as the Key Feature in the Biosynthesis of (+)-Otellione A,University of Minnesota, p20
- ↑ Houk, K. N., Lin, Y. T., & Brown, F. K. (1986). Evidence for the concerted mechanism of the Diels-Alder reaction of butadiene with ethylene. Journal of the American Chemical Society, 108(3), 554–6 doi:10.1021/ja00263a059
- ↑ Schmart, I. M. (2004). Endo- vs . Exo-Selectivity in Diels-Alder Reactions of Maleic Anhydride, 36(November), 81–82.
- ↑ 5.Fox, M. A., Cardona, R., & Kiwiet, N. J. (1987). Steric Effects vs. Secondary Orbital Overlap in Diels-Alder Reactions., (8), 1469–1474.