Rep:Mod:RS1111
Molecular Orbitals
MO1 is of energy -66.12596 a.u., and corresponds to the 1s orbital of Si, which is not involved in any significant bonding since it is so low in energy. It can be seen that the electron density is localised on the Si atom and that this MO is small, due to the strong effective nuclear charge experienced by these electrons, since silicon has 14 protons in its nucleus. By contrast, hydrogen has only 1 proton in its nucleus, therefore the nuclear charge experienced by its electron in a 1s orbital is much smaller, hence this orbital is a lot higher in energy than the 1s of silicon.
MO2 is of energy -5.28056 a.u., and corresponds to the 2s orbital of Si, which is also not involved in any significant bonding since it is also much lower in energy than the H 1s AOs. The electron density is more diffuse than the MO1, since the 2 electrons occupying this orbital are shielded from the nuclear charge of Si by the electrons in MO1, hence they experience a smaller effective nuclear charge.
MOs 3, 4 and 5 are degenerate MOs of energy -3.63858 a.u., corresponding to the 3 2p orbitals of Si, which are also too low in energy to be involved in bonding.
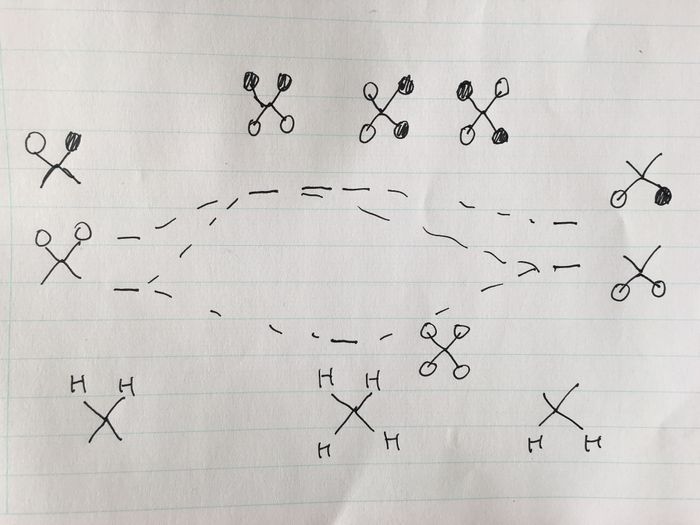
Atomic orbitals in the third, valence shell of Si are of similar energy to the hydrogen 1s orbitals, hence they can interact. MOs higher in energy can be rationalised by considering linear combinations of the valence orbitals of Si with the ligand group orbitals of a 'H4' fragment. A MO diagram can be constructed to determine these orbitals for the H4 fragment, as shown below (the lab demonstrator Becky assisted me in constructing this diagram).
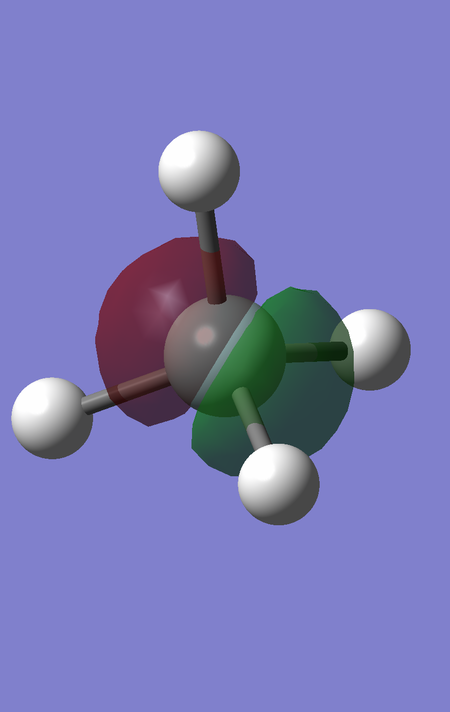
MO6 corresponds to the favourable, bonding interaction between the fully bonding fragment orbital for H4 and the 3s orbital of Si, and is of energy -0.54726 a.u.
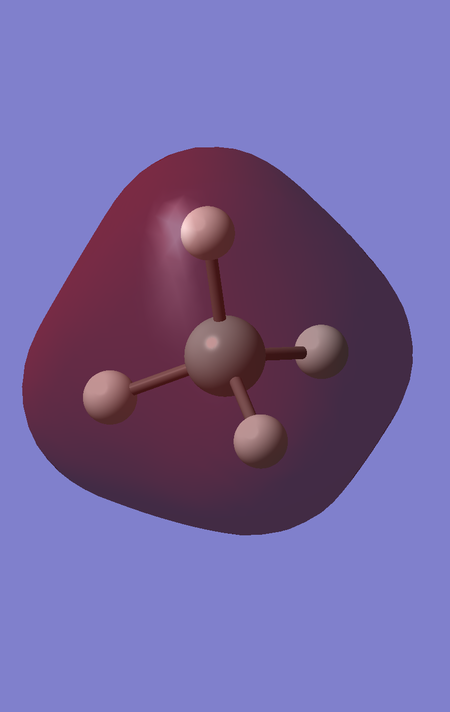
MOs 7,8 and 9 are degenerate, and correspond to the bonding interaction between the triply degenerate fragment orbitals of H4 with the 3 3p orbitals of Si. They are of energy -0.35184 a.u.
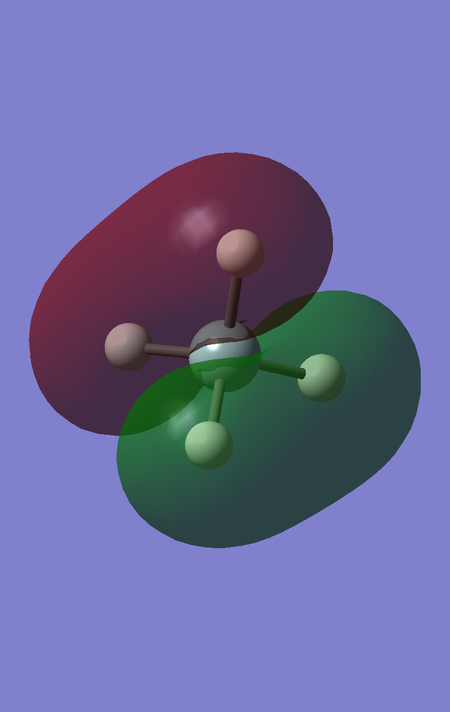
MOs 10, 11 and 12 are also degenerate; they correspond to the antibonding interaction between the triply degenerate fragment orbitals of H4 and the 3 3p orbitals of Si, and are hence higher in energy. These orbitals are the LUMOs of the SiH4 molecule, and the fact that there are 3 degenerate LUMOs spanning over the whole molecule reflects the fact that this tetrahedral molecule can be attacked by a nucleophile from almost any direction. The energy of these orbitals is 0.05053 a.u. The fact that this energy is greater than 0 reflects that these orbitals are antibonding, and that filling these orbitals would destabilise the SiH4 molecule.
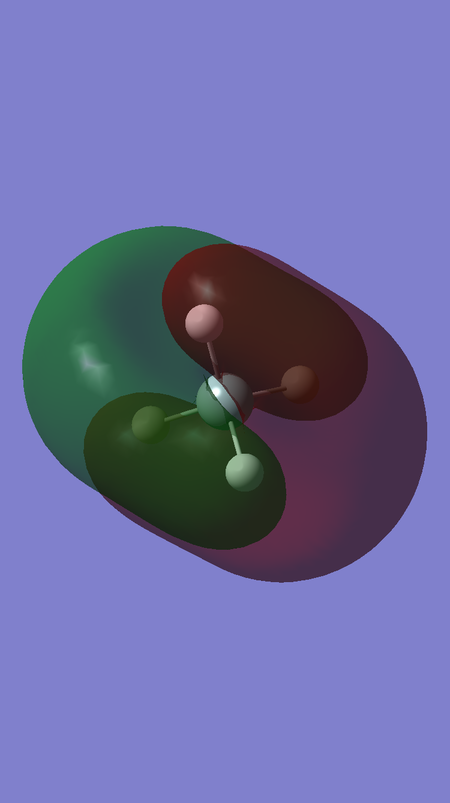
MO 13 corresponds to the antibonding interaction between the fully bonding fragment orbital for H4 and the 3s orbital of Si, and is of energy 0.12286 a.u.
The energies of MOs 6-13 can be rationalised by constructing a MO diagram modelling the interaction between the H4 fragment orbitals and the 3s and 3p orbitals of Si, as shown below (once again, the lab demonstrator Becky assisted me in constructing this diagram). Better overlap occurs between the fully bonding fragment of H4 and the 3s orbital of Si than between the triply degenerate fragment orbitals of H4 and the Si 3p orbitals. This will lead to stronger bonding and antibonding interactions, and therefore a greater difference in energy between the bonding and antibonding interaction, as can be seen in the MO diagram below.
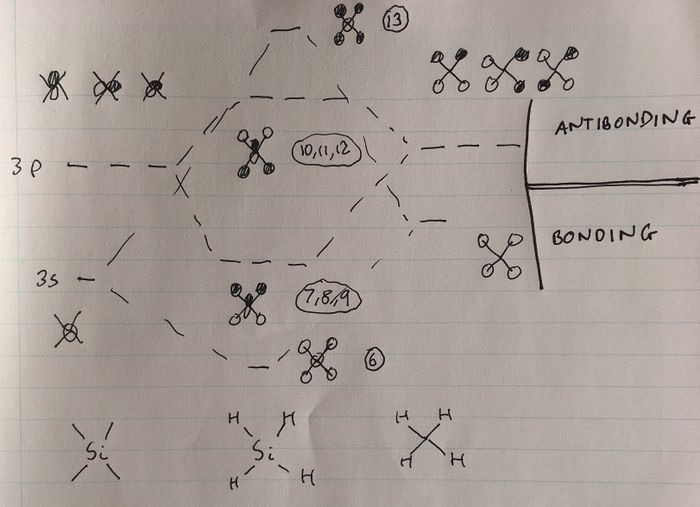
The fact that MOs 6 and 13 are more bonding and antibonding than MOs 7-9 and 10-12, respectively, can also be rationalised by looking at the distribution of electron density in the visualisations of these MOs. It can be seen that that there is greater electron density between the Si and H nuclei for MOs 6 and 13, compared to MOs 7-12.
