Rep:Mod:REN29
NH3 molecule
NH3 molecule |
The optimisation file is linked here
Optimisation results
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (RB3LYP) | -56.55776873 a.u. |
| RMS Gradient | 0.00000485 a.u. |
| Point group | C3v |
| N-H bond distance | 1.01798 Å |
| H-N-H bond angle | 105.741° |
Item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Vibrational modes
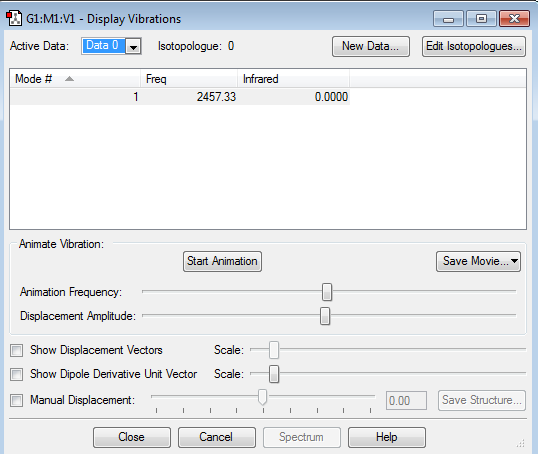
We expect to see 6 vibrational modes. (3N-6)
Modes 2 and 3 are degenerate, as are 5 and 6.
1, 2 and 3 are bending modes.
4, 5 and 6 are stretching modes.
4 is a highly symmetric mode.
1 is the umbrella mode.
Mode 5 and 6 would not be seen on the IR spectrum of ammonia as it has a very low intensity. Modes 2 and 3 are degenerate. Because of these two factors we would expect to see 3 bands in an experimental spectrum of gaseous ammonia.
Charge Analysis
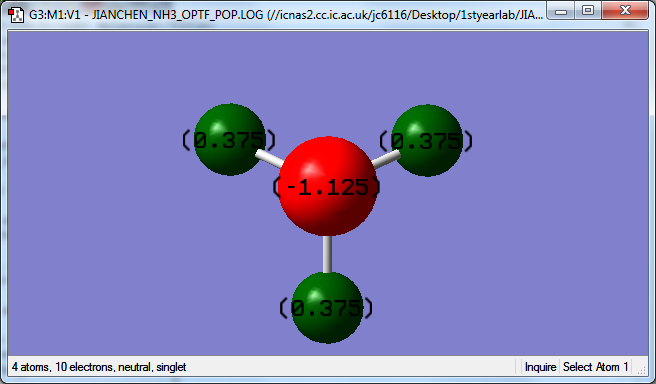
The charge on the N-atom is found to be -1.125.
The charge on the H-atoms is 0.375.
I would expect N to have a negative charge as it has a lone pair of electrons.
N is more electronegative than H, and thus draws electron density away towards itself.
H is more electropositive than N and therefore I would expect it to have a positive charge.
N2 molecule
N2 molecule |
The optimisation file is linked here
Optimisation results
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (RB3LYP) | -109.52412868 a.u. |
| RMS Gradient | 0.00000060 a.u. |
| Point group | D∞h |
| N-N bond distance | 1.10550 Å |
Item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Vibrational modes
H2 molecule
H2 molecule |
The optimisation file is liked to here
Optimisation results
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (RB3LYP) | -1.17853936 a.u. |
| RMS Gradient | 0.00000017 a.u. |
| Point group | D∞h |
| H-H bond distance | 0.74279 Å |
Item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Vibrational modes
The Haber-Bosch process
N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+ 3*E(H2)] = -0.0557907 a.u.
ΔE= -146.48 kJ/mol
The forward reaction is exothermic.
The ammonia product is more stable than the gaseous reactants.
F2 molecule
F2 molecule |
The optimisation file is linked here
Optimisation results
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (RB3LYP) | -199.49825218 a.u. |
| RMS Gradient | 0.00007365 a.u. |
| Point group | D∞h |
| F-F bond distance | 1.40281 Å |
Item table
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
Vibrational modes
One vibrational mode is observed which is as we would expect from 3N-5.
The F2 molecule is not IR active because it is homodinuclear it will have no change in dipole moment.
The two F atoms have no net charge.
The molecule is homodinuclear, and therefore the bond is non-polar.
Molecular orbitals
This image shows the molecular orbital formed by the two in phase 1s orbitals.
These are very deep in energy due to the large electronegativity of F.
Much of the electron density can been seen close the F nuclei.
There is little electron density along the internuclear axis and therefore this molecular orbital is not relevant for bonding.
This molecular orbital is the 1σg and is occupied by two electrons.
This image shows the molecular orbital formed by the two out of phase 1s orbitals.
Again, these orbitals are non-bonding as they are very deep in energy.
This molecular orbital is occupied by two electrons and it is called the 1σu* orbital.
This image shows the 3σg orbital, which is occupied by two electrons.
This bonding orbital is formed by the overlap of the in phase 2pz orbital of each F atom.
Two nodal planes are seen, one at each nucleus.
This image shows one of the HOMOs.
There are two HOMOs which are degenerate.
One is formed by the out of phase 2px atomic orbitals.
The other is formed by the overlap of the 2py atomic orbitals when they are out of phase.
Four electrons occupy these antibonding orbitals, with two electrons occupying each one.
These molecular orbitals are named the 1πg* orbitals.
There are two nodal planes to be seen, one of which bisects the internuclear axis and the other which runs along the internuclear axis.
This image depicts the LUMO - the 3σu* orbital.
This unoccupied anti-bonding orbital is formed by the out of phase 2pz atomic orbitals.
There are three nodal planes - one along the internuclear axis and one at each of the F nuclei.
H2SiO molecule
H2SiO molecule |
The optimisation file is linked here
Optimisation results
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (RB3LYP) | -365.90001403 a.u. |
| RMS Gradient | 0.00000941 a.u. |
| Point group | Cs |
| Si=O bond distance | 1.53172 Å |
| Si-H bond distance | 1.48652 Å |
| H-Si-O bond angle | 124.158° |
| H-Si-H bond angle | 111.686° |
The above values differ from experimentally determined values for the Si=O bond length, which was found to be 1.515 Å, and for the Si—H bond it was measured as 1.472 Å. [1]
Item table
Item Value Threshold Converged? Maximum Force 0.000023 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000017 0.001200 YES
Charge Analysis
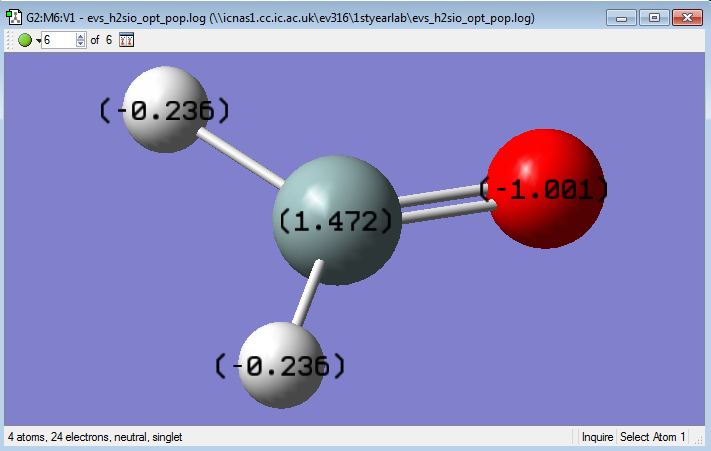
The Si atom has a +1.472 charge.
The O atom has a -1.001 charge.
The H atoms each have a charge of -0.236.
O and H are both more electronegative than Si.
The Si-O bond is strongly polarised due to the large electronegativity difference between O and Si.
The makes this molecule highly unstable and reactive.
References
- ↑ M. Bogey, B. Delcroix, A. Walters and J.-C. Guillemin, J. Mol. Spectrosc., 1996.