Rep:Mod:R1chie
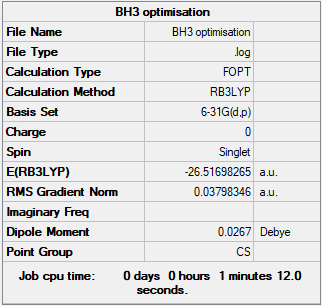
BH3 Molecule
Basis Set
B3LYP/6-31G(d.p.)
Summary Table
Item Table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000017 0.001800 YES
RMS Displacement 0.000011 0.001200 YES
Jmol 3D Model
BH3 Molecule |
Frequency Analysis
Low Frequencies Table
Low frequencies --- -0.7437 -0.4573 -0.0054 10.8204 14.8992 14.9177 Low frequencies --- 1163.0234 1213.2008 1213.2035
Frequency Link
Frequency file: Media:R1CHIE_BH3_FREQ.LOG
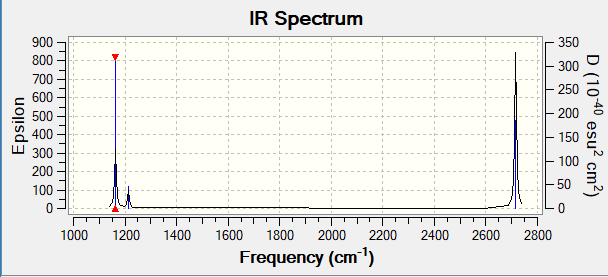
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92.5 | A2 | yes | out-of-plane bend |
| 1213 | 14.1 | E | yes | bend |
| 1213 | 14.1 | E | yes | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2715 | 126.3 | E | yes | asymmetric stretch |
| 2715 | 126.3 | E | yes | symmetric stretch |
IR spectrum for BH3
Questions
Q:In your wiki explain why are there less than six peaks in the spectrum, when there are obviously six vibrations.
A:Obviously, there exits six vibrational modes for BH3 but only three peaks present in IR spectrum. That is because bending modes 2 and 3 are degenerated and modes of stretching 5 and 6 are degenerated. Meanwhile, for mode 4, this vibration has no dipole moment, which means it is IR inactive. Therefore, there are only three peaks presented.
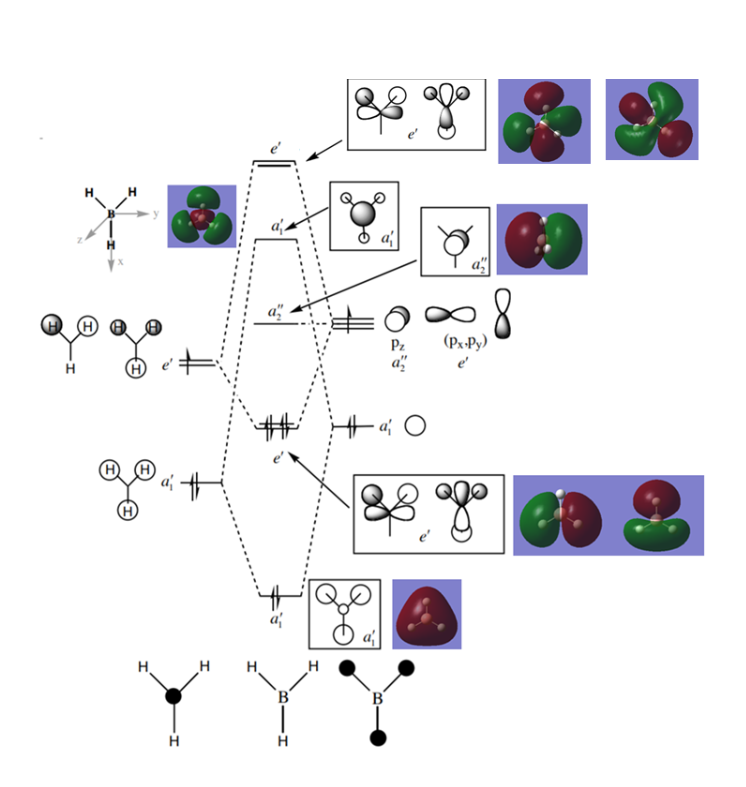
MO Diagram for BH3
Graph
Reference: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
Questions
Q:Are there any significant differences between the real and LCAO MOs? What does this say about the accuracy and usefulness of qualitative MO theory?
A:The LCAO MOs represents the time-averaged electron distribution around an molecule but in real, it is dynamic. The LCAO MOs of occupied and non-bonding orbitals are consistent with the real orbitals but for other unoccupied orbitals, the real and LCAO MOs are not perfectly matched.
Good inclusion of the calculated MOs onto the MOs diagram with the corresponding LCAOs. The LCAOs are just a drawn approximation and aren't really 'time-averaged', additionally, it's not clear if you're then implying that the calculated MOs are dynamic? However, the MOs are calculated using DFT where the electron-electron interaction is treated as an average interaction and aren't fully dynamic either. Smf115 (talk) 09:10, 30 May 2019 (BST)
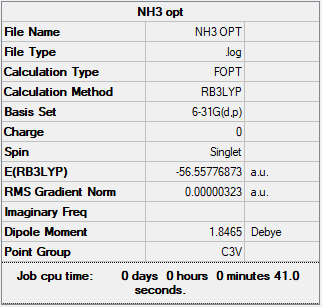
NH3 Molecule
Basis Set
B3LYP/6-31G(d.p.)
Summary Table
Item Table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Jmol 3D Model
NH3 Molecule |
Frequency Analysis
Low Frequencies Table
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
Frequency Link
Frequency file: Media:NH3_OPT_FRQ.LOG
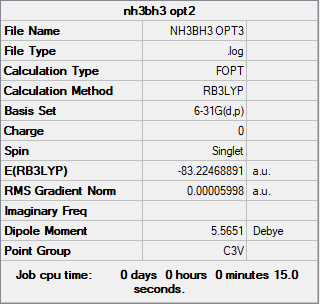
NH3BH3 Molecule
Basis Set
B3LYP/6-31G(d.p.)
Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000535 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Jmol 3D Model
NH3BH3 Molecule |
Frequency Analysis
Low Frequencies Table
Low frequencies --- -0.0252 -0.0032 0.0003 17.1228 17.1254 37.1306
Low frequencies --- 265.7808 632.2034 639.3480
Frequency Link
Frequency file: Media:NH3BH3_FRQ.LOG
Association Energy
Calculation
E(NH3)= -56.55776873 a.u. E(BH3)= -26.61532364 a.u. E(NH3BH3)= -83.22468890 a.u. Therefore, ΔE= E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22468890-(-56.55776873-26.61532364) a.u. = -0.05159653 a.u. = -0.05159653*2625.5 kJ/mol = -135.466689515 kJ/mol
Questions
Q:Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?
A:Rested on the calculation, the B-N dative bond is weak compared to the literature value of B-N covalent bond energy 389 KJ/mol.
Correct calculation but consider the accuracy of your final reported energy value. You've made a relevant comparison but literature values should always be referenced! Smf115 (talk) 09:12, 30 May 2019 (BST)
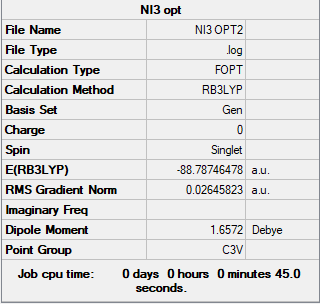
NI3 Molecule
Basis Set
B3LYP/Gen
Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000102 0.000450 YES RMS Force 0.000075 0.000300 YES Maximum Displacement 0.000667 0.001800 YES RMS Displacement 0.000490 0.001200 YES
Jmol 3D Model
NI3 Molecule |
Frequency Analysis
Low Frequencies Table
Low frequencies --- -80.9103 -80.9099 -73.9230 -0.0094 -0.0087 -0.0046 Low frequencies --- 126.2130 126.2170 175.1063
Frequency Link
Frequency file: Media:NI3_OPT2_FREQUENCY.LOG
Questions
Q: What is your optimised N-I distance?
A: The optimised N-I distance is measured to be 2.03 Å.
I think something has gone wrong when implementing your pseudopotential (PP) here as the energy and bond distance aren't right. In the frequency file you've submitted you have entered the PP wrong using 'lanl2mb' as the basis set rather than the 'Gen' keyword and then specifying the input seperately. Smf115 (talk) 09:16, 30 May 2019 (BST)
Mini Project
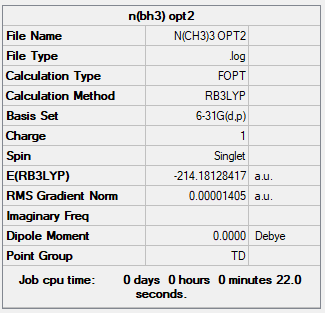
[N(CH3)4]+ Ion
Basis Set
B3LYP/6-31G(d.p.)
Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000284 0.001800 YES RMS Displacement 0.000119 0.001200 YES
Jmol 3D Model
[N(CH3)4]+ Ion |
Frequency Ananlysis
Low frequencies --- -0.0005 0.0004 0.0008 22.7319 22.7319 22.731
Low frequencies --- 189.0898 292.9370 292.9370
Frequency file: Media:N(CH3)3_FRQ3.LOG
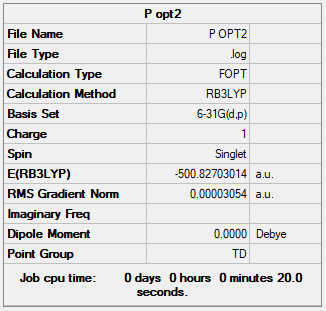
[P(CH3)4]+ Ion
Basis Set
B3LYP/6-31G(d.p.)
Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000966 0.001800 YES RMS Displacement 0.000385 0.001200 YES
Jmol 3D Model
[P(CH3)4]+ Ion |
Frequency Ananlysis
Low frequencies --- 0.0014 0.0014 0.0020 26.5719 26.5719 26.5719
Low frequencies --- 161.5135 195.9051 195.9051
Frequency file: Media:P_FRQ_4.LOG
Charge Distribution
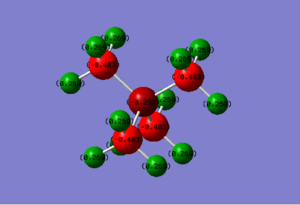
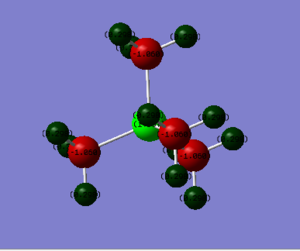
In traditional pictures, the formal charge is always to be considered to be located at the center atom which as N and P respectively. However, it can be only applied to[P(CH3)4]+] ion. As for [N(CH3)4]+], rested on the highly electronegativity of nitrogen atom, the negative charge is located around N and C atoms and the H attached to the carbon are positively charged.
In [N(CH3)4]+] ion:
Charge of N = -0.295
Charge of C = -0.483
Charge of H = +0.269
In [P(CH3)4]+] ion:
Charge of P = +1.664
Charge of C = -1.060
Charge of H = +0.298
Correct NBO charges calculated and clearly presented above. However, an equal colour range should have been used across both ILs to highlight the charge distribution. Your answer also sadly lacks any real analysis of the charges and you do not explain why the +1 traditional charge is calculated on the N in the formal picture, this section should have been a lot more developed. Smf115 (talk) 16:43, 30 May 2019 (BST)
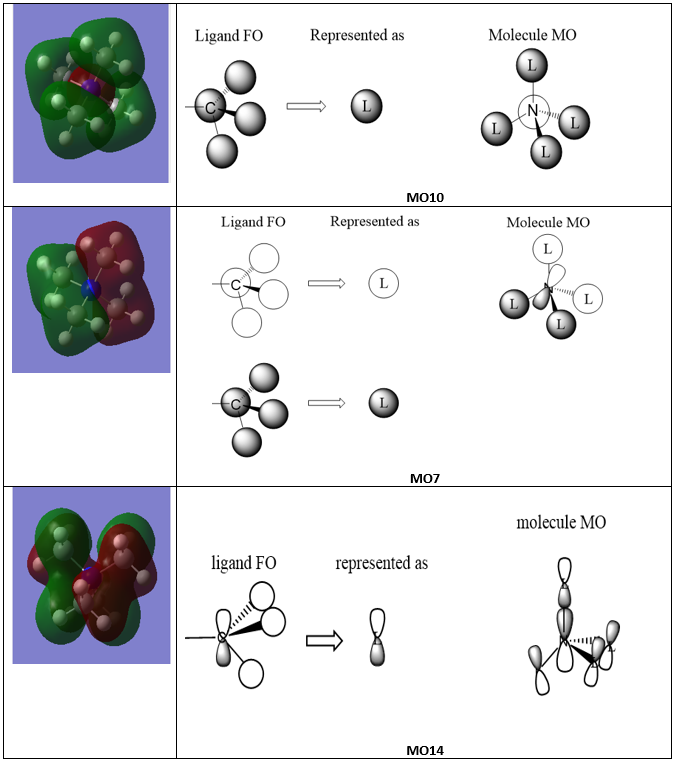
Molecular Orbitals for [N(CH3)4]+ Ion
Good attempt at constructing the FOs and LCAOs for the MOs selected. The FO for MO14 is incorrect and it might have been helpful to consider the BH3 MO diagram when trying to assign them. To improve, it would have also been good to seem the overall character of the MO identified and maybe some of the main interactions highlighted. Smf115 (talk) 16:48, 30 May 2019 (BST)
Overall, a decent attempt which could have done with more analysis in the project section. Smf115 (talk) 16:49, 30 May 2019 (BST)