Rep:Mod:Quantopia
Cope Rearrangement of Hexadiene
Optimisation of guess structures
A selection of structures were optimised and compared to the structures found in the appendix in the manual.[[1]].
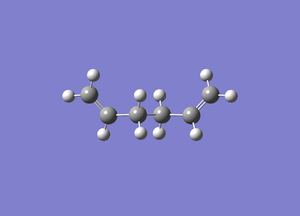
AntiPeriPlanar (APP)
Energy:-231.68165912
| Title | Result |
|---|---|
| File Name | hexa_ALF_anti |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy (au) | -231.69260236 |
| Gradient | 0.00001296 |
| Dipole Moment | 0.2021 |
| Point Group | C2 |
| Duration of Calculation | 18 seconds |
a jmol file can be found
This molecule matches the energy and symmetry of anti 1 in the manual.
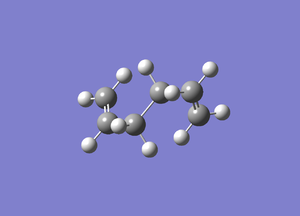
Gauche
Energy: -231.00983652
| Title | Result |
|---|---|
| File Name | Hexa_ALF_GAUCHE_2 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy (au) | -231.68771435 |
| Gradient | 0.00003625 |
| Dipole Moment | 0.4553 |
| Point Group | C2 |
| Duration of Calculation | 1 minute 1 second |
a jmol file can be found
The energy and symmetry match the gauche 1 structure in the manual.
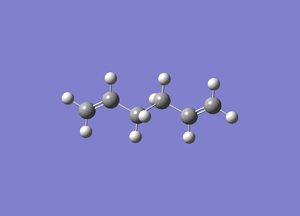
APP Ci
Energy: -231.68029455 Symmetry: Ci
| Title | Result |
|---|---|
| File Name | Hexa_ALF_Anti_2 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy (au) | -231.69253528 |
| Gradient | 0.00001891 |
| Dipole Moment | 0.000 |
| Point Group | C1/Ci |
| Duration of Calculation | 19 seconds |
A jmol file can be found
This matches the energy and symmetry of the anti 2 structure.
APP Ci Reopt
The above anti2 structure was reoptimised further with a better basis set.
| Title | Result |
|---|---|
| File Name | Hexa_ALF_Anti_3 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G |
| Final Energy (au) | -234.55971600 |
| Gradient | 0.00001343 |
| Dipole Moment | 0.000 |
| Point Group | C1/Ci |
| Duration of Calculation | 1 minute 16 seconds |
A jmol file can be found
Sum of electronic and zero-point Energies= -234.416221 Sum of electronic and thermal Energies= -234.408945 Sum of electronic and thermal Enthalpies= -234.408001 Sum of electronic and thermal Free Energies= -234.447765
Using this better basis set, the energy has dropped dramatically, showing the benefits of using better basis sets in your modelling.
Butadiene
Butadiene was optimised at the semi empirical AM1 level.
The MO's were then visualised from the checkpoint file File:Cis Buta ALF.chk
A jmol file can be found
MO's
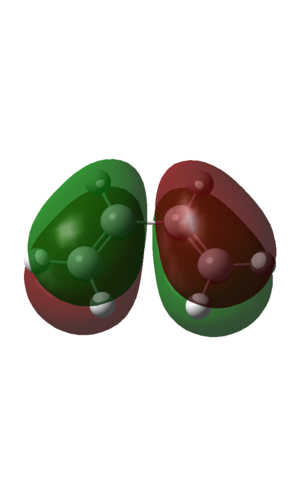
Homo
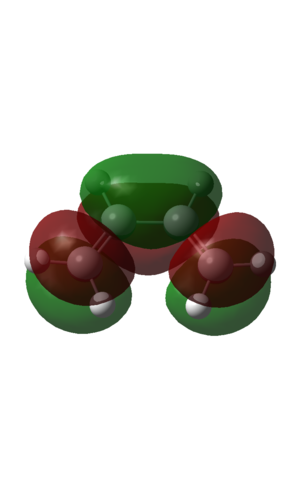
| HOMO | LUMO |
 |
|
| Two nodal planes in orbital. Antisymmetric with respect to phase | Three nodal planes in orbital. Symmetric with respect to phase. |
These MO's agree with the postulate that the HOMO is of the same symmetry as the HOMO ethene, and also the LUMOs.
Diels Alder transition state
Using this optimised structure of butadiene, the transition state in the diels alder cyclisation reaction between butadiene and ethene was modelled.
Optimisation
To form the transition state guess structure, the 2,2 bicycle was formed, two CH2 fragments were removed and two bonds were changed to dashed bonds. Double bonds were added where necessary, and the calculation was run. Logfile: File:BUTA ALF TRANS.LOG
Summary Table
| Title | Result |
|---|---|
| File Name | BUTA_ALF_TRANS |
| File Type | .log |
| Calculation Type | FTS |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy (au) | -231.60320856 |
| Gradient | 0.00001777 |
| Dipole Moment | 0.5753 |
| Point Group | C1 |
| Duration of Calculation | 1 minute 35 seconds |
A jmol file can be found
The optimisation has found a stationary point, so it has run to the stable minima.
Item Value Threshold Converged?
Maximum Force 0.000046 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000459 0.001800 YES
RMS Displacement 0.000109 0.001200 YES
Predicted change in Energy=-7.545022D-09
Optimization completed.
-- Stationary point found.
----------------------------
Geometry
The structure is shown here, the bond distances for the half formed C-C bonds are 2.10Å 3sf. The typical sigma bond bond lengths for an sp2 carbon to an sp3 is 1.507Å[1], and for sp2-sp2, 1.46Å[1]. For sp3 - sp3 1.53Å[1]. The typical double bond length for an sp2 carbon to another sp2 carbon is 1.316Å[1]. The van der Waals radius for carbon is 1.7Å[2]. Obviously the van der Waals contact distance is twice that: 3.4Å This means that our calculated value of 2.10Å sits two thirds of the way between vdW contact and a single bond.
| Type of Carbons | Length Å[1] |
|---|---|
| sp3 - sp3 | 1.53 |
| sp3 - sp2 | 1.507 |
| sp2 - sp2 | 1.46 |
| sp2 = sp2 | 1.316 |
Frequency Analysis
A frequency analysis was run on this optimised molecule. Logfile: File:BUTA ALF TRANS FREQ.LOG
Vibrations
There is one imaginary vibration at -818, which corresponds to the motion the carbons undergo while forming the bonds:
Comparing this to the lowest frequency positive vibration, which is a simple rotation, with the two molecules rotating in opposition to each other:
Molecular Orbitals
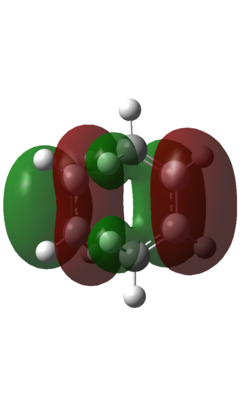
The HOMO of the transition state is shown below:
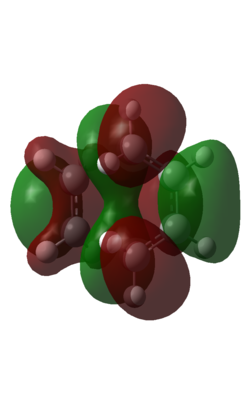
| HOMO | LUMO |
 |
|
| Three nodes in orbital. Symmetric with respect to phase | Four nodes in orbital. Symmetric with respect to phase. |
This shows that the LUMO of the butadiene and the HOMO of the ethene have reacted to form a symmetric orbital...obeying the rule that two orbitals of the same symmetry react to give two orbitals of the same symmetry.
Maleic Anhydride and Cyclohexadiene
Maleic anhydride reacts with cyclohexadiene to give a bicyclic system with either the endo isomer or the exo.
Optimisation of transition state
Endo
File:MALEIC ANHYDRIDE TS ALF.LOG
Results table
| Title | Result |
|---|---|
| File Name | MALEIC_ANHYDRIDE_TS_ALF |
| File Type | .log |
| Calculation Type | FTS |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy (au) | -605.61036823 |
| Gradient | 0.00000579 |
| Dipole Moment | 6.7141 |
| Point Group | C1 |
| Duration of Calculation | 13 minutes 24 seconds |
Item Value Threshold Converged?
Maximum Force 0.000019 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000338 0.001800 YES
RMS Displacement 0.000052 0.001200 YES
Predicted change in Energy=-3.129281D-09
Optimization completed.
-- Stationary point found.
Exo
Results table
| Title | Result |
|---|---|
| File Name | LAST_DITCH_ALF |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy (au) | -605.60359125 |
| Gradient | 0.00000720 |
| Dipole Moment | 5.9365 |
| Point Group | C1 |
| Duration of Calculation | 36 seconds |
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000475 0.001800 YES
RMS Displacement 0.000099 0.001200 YES
Predicted change in Energy=-4.942929D-09
Optimization completed.
-- Stationary point found.
Frequency Analysis
Endo
File:MALEIC ANHYDRIDE TS ALF FREQ.LOG
Exo
The log file for the optimisation doubles as the log file for frequency analysis as an opt+freq was run.
MO analysis
The HOMOs of the Endo and Exo structures are shown below.
| ENDO | EXO |
 |
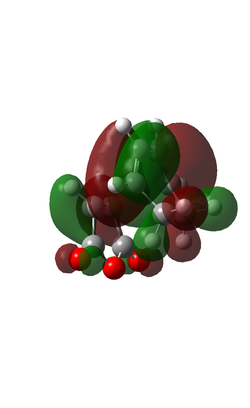
|
| Four nodes in orbital. Symmetric with respect to phase | Four nodes in orbital. Antisymmetric with respect to phase. |
Conclusion
From the visualised HOMO we can see that there is a nodal plane running between the -(C=O)-O-(C=O)- fragment and the rest of the system. This leads me to believe that the stereospecificity is not a result of the Secondary Orbital Interactions (SOI). This is backed up in papers that suggest that the endo is favoured, not because of orbital interactions, but because of solvent effects or hydrogen bonding, amongst other more common interactions[3]. The exo is more strained than the endo form, because the oxygen atoms are forced up against the hydrogens of the ch2 groups. This leads to more steric strain than is present in the endo form.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Frank H. Allen, Olga Kennard, David G. Watson, J. CHEM. SOC. PERKIN TRANS. , 1987,12, S1-S19 Cite error: Invalid
<ref>tag; name "F.Allen" defined multiple times with different content - ↑ A.Bondi, The Journal of Physical Chemistry, 1964, 68 (3), 441-451
- ↑ J.Garcia, J. Mayoral, L. Salvatella, Acc. Chem. Res. , 2000, 33, 658-664