Rep:Mod:QWE9630
Optimisation and Analysis of Molecules using Gaussian
The program Gaussian was used to optimise molecules to find the lowest energy structure of the molecules. From the optimised molecules, useful information such as bond lengths, vibrational modes and charges on the atoms were obtained. The molecular orbitals of the molecule can also be visualised to give a better understanding of the bonding in the molecule.
The reaction energy of the Haber-Bosch process was also calculated using the final energies of the molecules NH3, H2 and N2 obtained from optimisation.
NH3 (Ammonia)
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy/ au | -56.55776873 |
| RMS Gradient/ au | 0.00000485 |
| Point Group | C3v |
| N-H Bond Distance/ Å | 1.01798 |
| H-N-H Bond Angle/ o | 105.741 |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol dynamic image of the optimised ammonia molecule |
The optimisation file for NH3 is here.
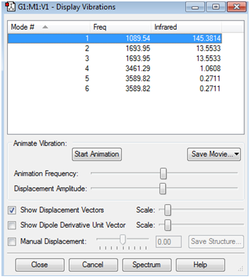
Vibrational Analysis
From the 3N-6 rule, it is expected that there will be 6 vibrational modes.
From the 'Display Vibrations' window, modes 2 and 3 are degenerate with a frequency of 1693.95 Hz, and modes 5 and 6 are degenerate with a frequency of 3589.82 Hz.
Modes 1,2 and 3 are bending vibrations, while modes 4,5 and 6 are bond stretching vibrations.
Modes 1 and 4 are highly symmetric, with mode 1 being known as the "umbrella" mode.
In the experimental spectrum of gaseous ammonia, it is expected that there would be 2 bands.
Charge Analysis
The charges on nitrogen and hydrogen are -1.125 and +0.375 respectively.
Nitrogen is more electronegative than hydrogen and so tends to attract electrons towards it more than hydrogen. It is therefore expected that nitrogen will have a negative charge and hydrogen will have a positive charge.
H2 (Hydrogen)
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy/ au | -1.17853929 |
| RMS Gradient/ au | 0.00013423 |
| Point Group | D∞h |
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
Jmol dynamic image of the optimised hydrogen molecule |
The optimisation file for H2 is here.
Vibrational mode frequency= 4470.30 Hz
N2 (Nitrogen)
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy/ au | -109.52412868 |
| RMS Gradient/ au | 0.00000437 |
| Point Group | D∞h |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol dynamic image of the optimised nitrogen molecule |
The optimisation file for N2 is here.
Vibrational mode frequency= 2457.31 Hz
Haber-Bosch Reaction Energy Calculation
The Haber-Bosch process involves the conversion of N2 and H2 to NH3, which is used to produce fertilisers. The energy difference for the chemical reaction N2 + 3H2 -> 2NH3 was calculated as follows:
E(NH3)= -56.55776873 au
2*E(NH3)= -113.1155375 au
E(N2)= -109.52412868 au
E(H2)= -1.17853929 au
3*E(H2)= -3.53561787 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -146.4791392 kJ/mol
The reaction has a negative energy difference and hence the ammonia product is more stable than the gaseous reactants.
SiH4 (Silane)
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy/ au | -291.88802760 |
| RMS Gradient/ au | 0.00000002 |
| Point Group | Td |
| Si-H Bond Distance/ Å | 1.48485 |
| H-Si-H Bond Angle/ o | 109.471 |
The H-Si-H bond angle suggests that SiH4 has a tetrahedral shape. The Si-H bond length in silane is greater than the C-H bond length in methane because silicon is a larger atom than carbon and so there is less effective overlap of the 3s and 3p orbitals of silicon with the 1s orbital of hydrogen.
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol dynamic image of the optimised silane molecule |
The optimisation file for SiH4 is here.
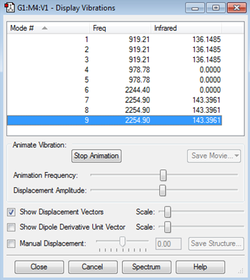
Vibrational Analysis
From the 3N-6 rule, it is expected that there will be 9 vibrational modes.
The degenerate stretching modes 7-9 have a vibrational frequency of 2254.90 Hz while the degenerate bending modes 1-3 have a vibrational frequency of 919.21 Hz.
Modes 4-6 do not involve a change in the dipole moment and are not IR active (they will not produce a band in the IR spectrum). Hence in the IR spectrum of SiH4, it is expected that there will only be two bands arising from the two degenerate modes.
Charge Analysis
The charges on silicon and hydrogen are +0.629 and -0.157 respectively.
This is because hydrogen is more electronegative than silicon and therefore will tend to attract electrons towards it more strongly than silicon.
Molecular Orbitals
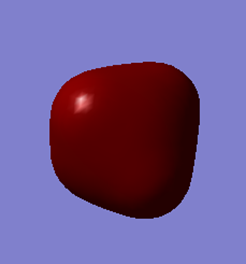
The occupied core 2s non-bonding molecular orbital of Si. It is deep in energy (-5.28 au) and is not involved in chemical bonding; the orbital is held tightly to the Si nucleus and does not overlap with the 1s atomic orbital of the hydrogen atoms.
The occupied molecular orbital formed from the combination of the valence 3s orbital of Si and the 4 1s orbitals of the 4 hydrogen atoms. This bonding molecular orbital is involved in chemical bonding as there is a good overlap of the silicon 3s atomic orbital and the hydrogen 1s atomic orbitals.
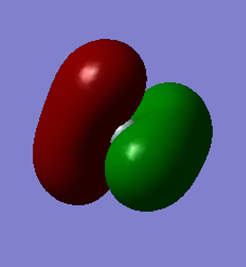
The occupied molecular orbital formed from the combination of the valence 3p orbital of Si and the 4 1s orbitals of the 4 hydrogen atoms. As silicon has 3 degenerate orthogonal 3p orbitals (3px, 3py, 3pz), there are 3 of these molecular orbitals of the same energy (-0.352 au). These bonding orbitals are the HOMO and are involved in chemical bonding.
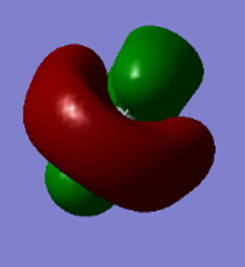
The antibonding combination of the valence 3p orbital of Si and the 4 1s orbitals of the 4 hydrogen atoms. There will be 3 of these degenerate antibonding molecular orbitals due to the same reason as above with the bonding combination. These orbitals (+0.051 au) are unoccupied and are the LUMO.
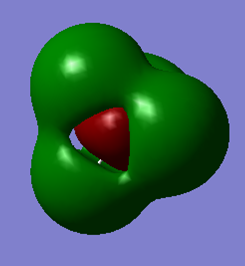
The antibonding combination of the valence 3s orbital of Si and the 4 1s orbitals of the 4 hydrogen atoms. This unoccupied molecular orbital is high in energy (+0.123 au).
H2CO (Formaldehyde)
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy/ au | -114.50319933 |
| RMS Gradient/ au | 0.00006910 |
| Point Group | C2v |
Item Value Threshold Converged? Maximum Force 0.000182 0.000450 YES RMS Force 0.000080 0.000300 YES Maximum Displacement 0.000231 0.001800 YES RMS Displacement 0.000142 0.001200 YES
Jmol dynamic image of the optimised formaldehyde molecule |
The optimisation file for H2CO is here.
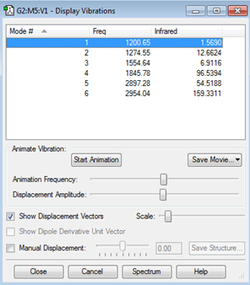
Vibrational Analysis
From the 3N-6 rule, it is expected that there will be 6 vibrational modes.
There are no degenerate vibrational modes.
Charge Analysis
The charges on oxygen, carbon and hydrogen are -0.494, +0.221 and +0.137 respectively. There is a dipole in the molecule, with a δ+ carbon and a δ- oxygen.
Oxygen is more electronegative than carbon, and therefore oxygen has a negative charge while carbon has a positive charge. The electronegativities of hydrogen and carbon are similar and so there isn't a large charge difference between the two atoms.
