Rep:Mod:POL234
Appearance
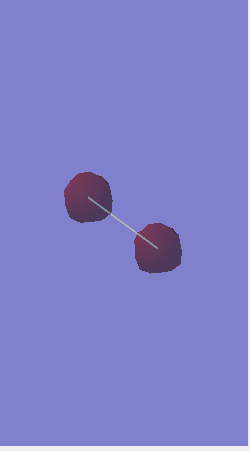
NH3 molecule
Information
Molecule name Ammonia Calculation Method RB3LYP Basis set 6-31G(d.p) Final energy -56.55776873 RMS gradient 0.00000485 Point group C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
N-H bond length 1.01798 Å Bond angle is 37.129 degrees
Image of NH33 Molecule
NH3 Molecule |
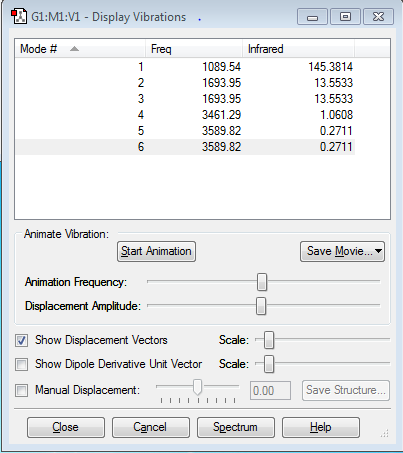
Vibrations of Nh3
Questions
1. From the 3N-6 rule we expect there to be 6 vibrational nodes as there are 4 atoms in the ammonia molecule. 2. Vibrational modes that are degenerate are pairs 2 and 3 and 5 and 6. 3. Modes 1,2 and 3 are bending vibrations and modes 4,5 and 6 are stretching vibrations. 4. Mode 4 is highly symmetric 5.Mode 1 is known as the umbrella mode 6.You would expect to see 2 bands.
The charge on the N atom is -1.125 and the charge on the H atoms is 0.375. You would expect this as the nitrogen atom is more electronegative then the hydrogen atom and so would have an increased electron density surrounding it giving it a higher negative charge. The hydrogen has less electrons surrounding it but only one proton so has a small positive charge.
N2 molecule
Information
Molecule Nitrogen Calculation Method RB3LYP Basis set 6-31G(d.p) Final energy -109.52412868 RMS gradient 0.00000017 Point group D*H
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Bond length 1.1055 Å
Image of N2
N2 molecule |
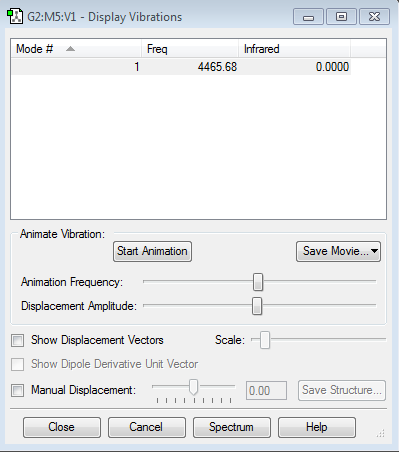
Vibrations of N2
This shows that the molecule is optimized because there are no negative vibrational energies.
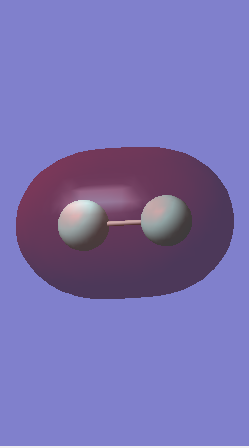
H2 molecule
Information
Molecule H<sub>2</sub> Calculation Method RB3LYP Basis set 6-31G(d.p) Final energy -1.17853936 Point group D*H
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Bond length 0.074279 Å
Image of H2 molecule
Hydrogen |
Virbrations of H2
This shows that the molecule is fully optimized because there are no negative vibrational modes.
Reaction energies
E(NH3)= -56.55776873 2*E(NH3)= -113.115537 E(N2)= -109.52412868 E(H2)= -1.17853936 3*E(H2)= -3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.055791 This is -146.48 kj/mol, therefore the ammonia product is the most stable as the reaction is exothermic so energy is released into the environment.. This figure is far off from one found in the literature* of -45.93kJ/mol this is due to the fact that they are measured under different conditions. *J. Chem. Soc., Trans., 1903,83, 1168-1184 DOI: 10.1039/CT9038301168
F2
Information
Molecule F<sub>2</sub> Calculation Method RB3LYP Basis set 6-31G(d.p) Final energy -199.49825218 RMS gradient 0.00007365 Point group D*H
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
Bond length 1.40281 Å. A literature value gives 1.417 Å. This is very close to the value obtained using Gaussian. http://www.wiredchemist.com/chemistry/data/fluorine-iodine-compounds
Image
Fluorine molecule |
Vibrations
There a no negative vibrational energies which shows that the molecule is fully optimized.
Atomic Charges
There is no charge on either of the fluorine atoms as it is a homonuclear diatomic molecule and so share evenly the electrons in the compound.
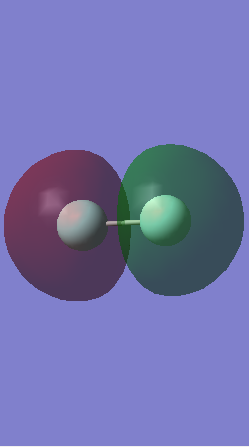
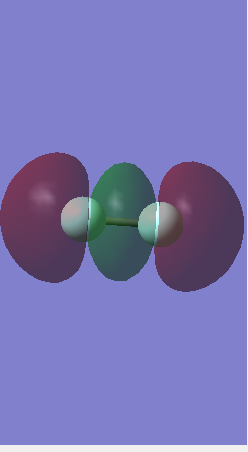
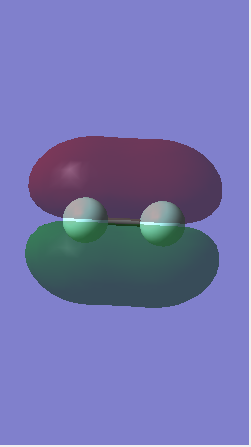
Molecular Orbitals
This is the 1s non bonding molecular orbital, it is non bonding because there is no overlap between the orbitals. The atomic orbitals are in phase and the orbital is so small it is found inside the fluorine atom. It is very deep in energy as they are core molecular orbitals.
This is the bonding sigma orbital. It is formed from the valence 2s atomic orbitals, these overlap more strongly and have a higher energy of -1.337 atomic units. It is very much involved in chemical bonding as it is the valence shell.
This is the anti bonding sigma* orbital and it has a higher energy than the sigma orbital as the s atomic orbitals do not overlap. The energy difference between the bonding and anti bonding orbital is greater than the 1s orbitals because the molecules in the bonding orbital overlap more.
This is a sigma bonding orbital made from atomic p orbitals overlapping along the bond. Its has a higher energy than the s orbitals of -0.58753 atomic units.
This is the pi bonding molecular orbital made from p atomic orbitals perpendicular to the plane of the bond. All of above orbitals are occupied.