Rep:Mod:Oxyt11
Project Molecule Analysis
The molecules were created using a program called gaussview. Optimisation is carried out on each molecule to ensure the information obtained is as accurate as possible. The energy , charge distribution, molecular structure, bond vibration and molecular orbitals are analysed and discussed.
NH3 molecule
Key Information
The molecules were created and their main properties were observed and analysed using program called gaussview. Optimisation is carried out on each molecule to ensure the information obtained is as accurate as possible.
Gaussian Calculation Summary
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -56.55776873 a.u.
RMS Gradient Norm = 0.00000176 a.u.
Point Group = C3V
Bond Length of N-H = 1.01798 Angstrom(Å)
Bond Angle of H-N-H = 105.741°
Item Table
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000028 0.001800 YES
RMS Displacement 0.000017 0.001200 YES
Predicted change in Energy=-1.261292D-10
Optimization completed.
-- Stationary point found.
Image of the molecule
Ammonia |
Click here for optimisation file.
Display Vibration Window
IR Analysis
From 3N-6 rule, 6 modes are expected from NH3 vibration (N=4)
From the display vibration window, it is observed that there are two degenerate sets of vibration which are mode 2 and 3 as one set and mode 4, 5, and 6 as the other set.
Bending Vibration : mode 1, 2, 3
Stretching Vibration : mode 4, 5, 6
Highly Symmetric Vibration : mode 4
Umbrella Mode : mode 1
In experimental spectrum of gaseous ammonia, only 2 bands are expected to be observed. This is because the intensity of vibration mode 4, 5, and 6 are too low to be detected due to small change in dipole during vibration. Among the detected mode 1, 2, and 3, mode 2 and 3 have similar frequency thus they only show as one band.
IR Spectrum of Molecule
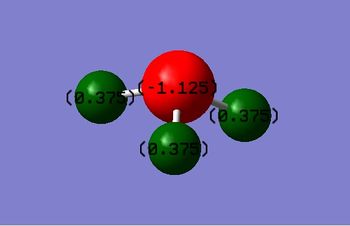
Charge Analysis
Charge on nitrogen(N): -1.125
Charge on hydrogen(H): 0.375
Nitrogen is a more electronegative species compared to hydrogen. Hence, N attracts the electron densities towards itself causing it has slightly negative in charge, leaving the three hydrogen atoms with delta positive charges.
Optimised H2 Molecule
Key Information
Gaussian Calculation Summary
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -1.17853936 a.u.
RMS Gradient Norm = 0.00000017 a.u.
Point Group = D∞h
Bond Distance of H-H = 0.74279 Å
Bond Angle of H-H = 180°
Item Table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
Image of Molecule
Hydrogen |
Click here for optimisation file.
Frequency of H2
The H2 single bond vibration is 4465.68 cm-1. This vibration is IR inactive as there is no change of dipole moment between two similar atoms during bond vibration.
Optimised N2 Molecule
Key Information
Gaussian Calculation Summary
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -109.52412868 a.u.
RMS Gradient Norm = 0.00000060 a.u.
Point Group = D∞h
Bond Length of N2 = 1.10550 Å
Bond Angle = 180°
Item Table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401023D-13
Optimization completed.
-- Stationary point found.
Image of Molecule
Nitrogen |
Click here for optimisation file.
Frequency of N2
The N2 single bond vibration is 2457.33 cm-1. This vibration is IR inactive as there is no change of dipole moment between two similar atoms during bond vibration.
Haber-Bosch process
Energy for the reaction of N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u. / -146.82 kJmol-1
Conclusion: The reaction is exothermic as heat energy is released (negative delta E), thus the ammonia product is more stable compared to the gaseous reactants.
Molecule of My Choice : Carbon Monoxide ( CO )
Key Information
Gaussian Calculation Summary
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -113.30945314 a.u.
RMS Gradient Norm = 0.00000433 a.u.
Point Group = C∞v
Bond Length of CO Triple Bond = 1.13794 Å
Bond Angle of CO = 180° (linear molecule)
Item Table
Item Value Threshold Converged?
Maximum Force 0.000007 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000003 0.001800 YES
RMS Displacement 0.000004 0.001200 YES
Predicted change in Energy=-2.221220D-11
Optimization completed.
-- Stationary point found.
Image of the molecule
Carbon Monoxide |
Click here for optimisation file.
IR Analysis
From 3N-6 rule, 1 modes are expected from CO triple bond vibration (N=2).
It is observed that there is only one vibration mode available for the CO molecules as it is linear and diatomic.
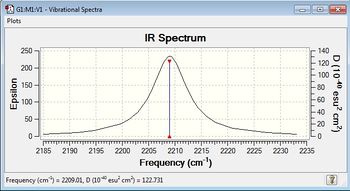
The vibrational frequency is 2209.01 cm-1 which results from the stretching of bond.
IR Spectrum of Molecule
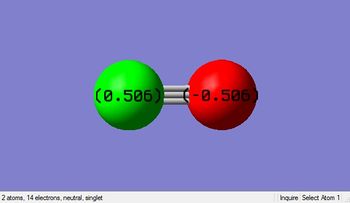
Charge Analysis
Charge on Carbon(C): 0.506
Charge on Oxygen(O): -0.506
Oxygen is a more electronegative species compared to hydrogen. Hence, O attract the electron densities towards itself causing it has slightly negative in charge, leaving the carbon with a delta positive charge.
Molecular Orbital of CO[1]
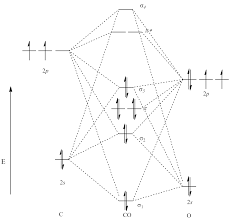
From the MO diagram, it is observed that the energy level of 2p σ MO is higher than the two degenerate π bonds. This is because of the mixing of 2s σ and 2p σ which is closed in energy.
Image of the Molecular Orbital
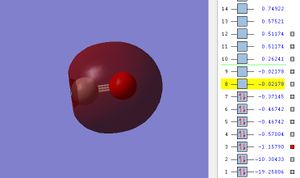
The potential energy of 2s σ MO is -1.15790.
From diagram, it is shown that there is an uneven distribution of electron density between C and O. Since oxygen(O) is more electronegative than carbon, its 2s atomic orbital is located at lower energy level. Thus, O has higher contribution in the sigma bonding MO and it hold greater proportion of electron density.
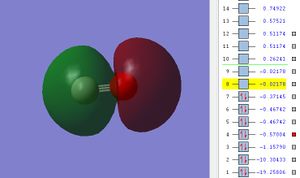
The potential energy of 2s σ* MO is -0.57004.
In the 2s σ* MO, the uneven distribution of electron density between C and O can also be explained due to the difference in atomic orbitals energy levels of C and O. Carbon(C) is less electronegative than carbon, its 2s atomic orbital is located at higher energy level. Thus, C contributes more in the higher energy sigma* bonding MO and it hold greater proportion of electron density.
Cartesian Plane[2]
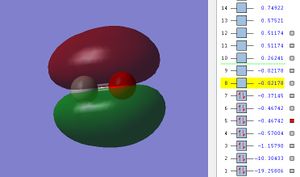
The potential energy of 2p π MO is -0.46742.
There are 2 π bond formed in the CO molecules due to the side way overlaps of 2 sets of 2p orbitals from C and O, the 2p orbital must be in correct orientation (example : px with px) to overlap. The energy of the two 2π orbitals are degenerates ( see diagram MO energy 5 and 6). The two π MO are orthogonal to each other according to cartesian plane.
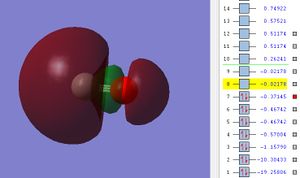
The potential energy of 2p σ MO is -0.37145.
This is the σ bond formed from the heads on overlap between two 2p atomic orbitals from C and O with correct orientation in phase. HOMO stands for highest occupied molecular orbital which is the highest energy molecular orbital that is filled with electron.
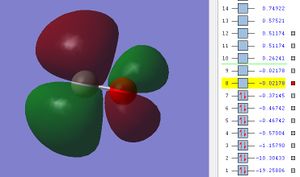
The potential energy of 2p π* MO is -0.02178.
This is the π* bond formed from the side on overlap between two 2p atomic orbitals from C and O with correct orientation out of phase. LUMO stands for lowest unoccupied molecular orbital which is the empty molecular orbital with lowest energy level.
Independence : CH4 Molecule
Key Information
Gaussian Calculation Summary
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -40.52401404 a.u.
RMS Gradient Norm = 0.00003263 a.u.
Point Group = Td
Bond Length of C-H = 1.09197 Å
Bond Angle of H-C-H = 109.471°
Item Table
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Predicted change in Energy=-2.256043D-08
Optimization completed.
-- Stationary point found.
Image of the molecule
Methane |
Click here for optimisation file.
Display Vibration Window
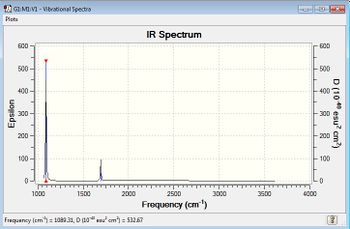
IR Spectrum of Molecule
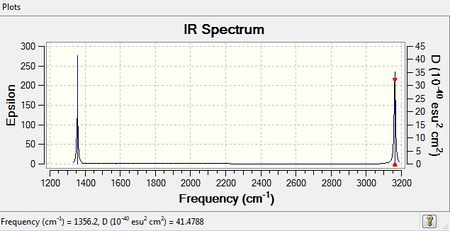
Frequency Analysis of CH4
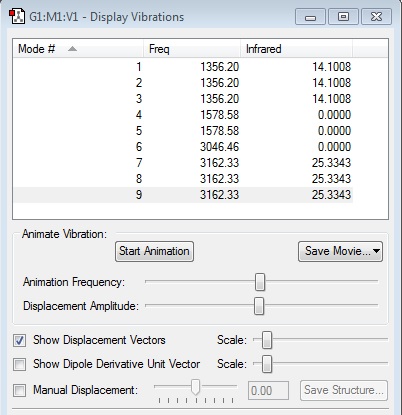
From 3N-6 rule, 9 modes are expected from CH4 vibration (N=5)
However, there are only 2 bands shown from the IR spectrum, which is the C-H bond bending at frequency 1256.2 cm-1 and stretching 3162.33 cm-1 . From the IR spectrum, vibration mode 1 , 2, and 3 are in the same energy so they only show as one peak;whereas vibration mode 7, 8, and 9 are the same energy, thus also show as one peak. There are no peaks show for vibration mode 4, 5, and 6 because there are no change in dipole moment in CH4 to be detected. This is be proved from the zero intensity. Bond stretching is at higher energy than bond bending.
The presence of only two bands in IR spectrum is due to the sp3 hybridisation of the carbon 2s and three 2p orbitals to form 4 degenerate sp3 atomic orbitals. Thus, only one type of C-H bond is observed. This gives CH4 tetrahedral geometry and Td point group with the bond angle of approximate to 109.5°.
sp3 Hybridisation[3]
Charge Analysis
Charge on carbon(C) = -0.930
Charge on hydrogen(H) = 0.233
Carbon is more electronegative compared to hydrogen. Hence, C attracts the electron densities towards itself causing it has slightly negative in charge, leaving the four hydrogen atoms with delta positive charges.
Molecular Orbitals of CH4
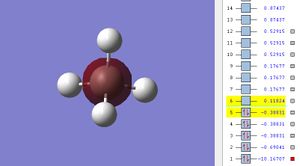
The potential energy is -10.16707 which is the lowest energy MO.
The electron contribution is only from the 1s2 of C.

The potential energy is -0.69041.
The 4 Hs' 1s atomic orbital(AO) overlap contructively with C's 2s AO. The electron density is contributed from the four H's 1s2 and C's 2s2.
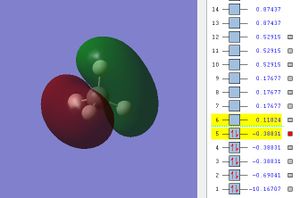
The potential energy of HOMO is -0.38831.
This MO is formed from the overlap of C's 2p AO and the four Hs' 1s AO. As C's 2px 2py, 2pz are degenerate and each of the 2p sub-orbital overlap with the four Hs' 1s AO, thus we obtained three 3σ bonding MO with the same energy that are orthogonal to each other.
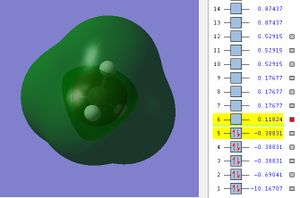
The potential energy of LUM0 is 0.11824.
This MO results from the destructive overlap of C's 2s and Hs' 1s AOs.
Reference
[1] Media:http://chem-is-you.blogspot.com/2013/02/fundamentals-of-molecular-bonding.html
[2] Media:http://www.zedism.com/pamelajaeger/defined.html
[3] Media:http://www.chemguide.co.uk/basicorg/bonding/methane.html