Rep:Mod:OJA692728
Part 1
The Hydrogenation of Cyclopentadiene Dimer
Using Computational Methods the stability of the kinetic and thermodynamic products of cyclopentadiene (CPD) dimerisation have been calculated. Using Avagadro to optimize both Molecule 1 and Molecule 2 with a MMFF94(s) force field the energies of the compounds has been calculated in kcal/mol and are shown in Table 1. From this it can be seen that product 1 is thermodynamically more stable and is lower in energy by ~3 kcal/mol. However, despite being higher in energy, the Kinetic product 2 is the one most observed when this dimerisation takes place. This leads one to assume that the reaction must be controlled by kinetics as opposed to thermodynamics meaning the transition state of the reaction to form 2 is lower in energy than that of 1.
| Energy (kcal/mol) | 1 (Exo) | 2 (Endo) |
|---|---|---|
| Bond Stretching | 3.54305 | 3.46800 |
| Angle Bending | 30.77276 | 33.18902 |
| Torsional | -2.73088 | -2.94965 |
| van der Waals | 12.80147 | 12.35890 |
| Electrostatic | 13.01367 | 14.18474 |
| Total | 55.37342 | 58.19067 |
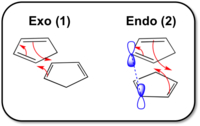
One explanation of this phenomena is the Secondary Orbital Interactions (SOIs) present in the endo (2) transition state which are not in the exo form. From the diagrams in Figure 1 this interaction can be seen; the mechanistic arrows are in red and the proposed SOIs of the Carbon pz orbitals in the endo form are in blue. This added interaction stabilizes the endo transition state as the two orbitals keep the CPD rings in position during the reaction and the part combination of the orbitals contributes to the lowering of energy for the entire system.
However there is another theory to explain the favoring of the endo product and hence why the reaction is kinetically controlled. By analyzing the endo and exo transition state and interesting feature regarding the individual stabilities of the Carbons and Hydrogens has been unearthed. [1]It can be argued that is the increased stabilisation of the Hydorgen atoms on both CPD rings that leads to a lowering of the energy barrier to form the endo product. As the sp2 carbons rehybridize to sp3 charge is transferred from the carbon to the hydrogen thus lowering it in energy. In the endo form the stabilization of the hydrogens is reflected in the second ring whereas in the exo TS this relationship is asymmetric thus leaving it unbalanced and as a whole transition state less stable. The pronunciation of Carbon sp2 to sp3 rehybridization is greater in the endo TS leading to a greater charge transfer and thus a greater stabilization. The stabilization energies calculated in this paper can be seen in Table 2.
| Endo | Exo | |||
|---|---|---|---|---|
| CPD | CPD' | CPD | CPD' | |
| Σ(ΔEC) | 36.73 | 36.73 | 17.03 | 50.34 |
| Σ(ΔEH) | -28.10 | -28.10 | -19.32 | -27.12 |
Molecule 3 and Molecule 4, the two hydrogenation products of the CPD dimer, were also evaluated using a similar method as above in order to establish which product is the result of thermodynamic and/or kinetic control. The results of these calculations can be seen in Table 3.
| Energy | 3 | 4 |
|---|---|---|
| Bond Stretching | 3.30608 | 2.82311 |
| Angle Bending | 30.85293 | 24.68536 |
| Torsional | 0.07367 | -0.37840 |
| van der Waals | 13.28027 | 10.63731 |
| Electrostatic | 5.12099 | 5.14702 |
| Total | 50.72279 | 41.25749 |
It can be concluded that that 4 is the thermodynamic product of the hydrogenation of the CPD dimer as it is lower in energy than 3. From the data in Table 3 it can be seen that both the angle bending and bond stretching energies are greater in molecule 3 which can be put down to the increased ring strain in the 6-membered ring. It has a bridging methyl group and a double bond which will both but a considerable amount of strain on the ring leading to it deviating from the expected 'norm' more. This in contrast to 4 which has the two limiting features spread across the whole molecule, and this is reflected in the negative torsional energy reported for the molecule. The electrostatic energy is almost the same as the molecules have the same empirical formula and basic structure so no differences in electrostatic energies was predicted. Another interesting feature is the position of the groups alongside the double bond in each molecule; looking along these bonds it can be seen that the hydrogens in 3 are in an eclipsed form whereas in molecule 4 they are gauche. This indicates that 4 is more stable than 3 as there is less steric hindrance between the groups adjacent to the double bonds. All of these factors contribute to 4 being lower in energy and thus the thermodynamic product of the hydrogenation of the CPD dimer.
From the data collected the kinetic product of the reaction can not be determined, as although 4 is the thermodynamic product it can still also be the kinetic product and due to the lack of conclusive data it would be wrong to assign either 3 or 4 as the kinetic product of this reaction.
Atropisomerism
By using the MMFF94s force field the energies of Molecule 9 and Molecule 10 can be calculated in order to determine which is the more stable conformer. The C6 ring in Molecule 9 was modelled as a twist boat and in Molecule 10 as a chair to be in line with the findings in the literature[2]. It can be seen that Molecule 10 is the thermodynamically stable of the two by evaluating the Total energies, in Table 4 as it is ~8 kcal/mol lower in energy than Molecule 9. Interestingly when molecule 10 was in the opposite chair form the energy was much higher, at approximately 180kcal/mol. Other configurations of the C6 ring were tried in order to determine if there was one in a lower energy but the ones laid out by Paquette[2] are the lowest forms of both of the molecules although the correct directionality of the ring has to be taken into consideration.
Apart from the configuration of the C6 ring the difference in these two molecules is where the carbonyl group points and the conformation of he C6 ring is arguably determined by this positioning as well in order to relieve the added strain. In molecule 9 the carbonyl group is forced into the same plane as the bridging iso propyl group leading to a larger torsional energy deviation as well as angle bending. Whereas in molecule 10 the carbonyl and bridging group are in opposite planes relieving this strain and hence there is a smaller deviation from the ideal.
In both of these compounds the alkene reacts unusually slowly considering the ring strain and reactivity of the double bond. However this can be put down to the molecules being hyper-stable, where the parent alkane is higher in energy than the corresponding alkene. This phenomena has been observed in molecules where a double bond lies adjacent to a bridgehead but as of yet there is not enough evidence to provide an explanation for why this happens. Maier and Schleyer[3] have observed hyper-stability in different cyclic alkenes with differing bridgehead sizes.
| Energy | Molecule 9 | Molecule 10 |
|---|---|---|
| Bond Stretching | 13.39848 | 12.69138 |
| Angle Bending | 59.85832 | 53.18714 |
| Stretch Bending | 0.09336 | -0.58690 |
| Torsional | 6.92784 | 6.11050 |
| Out-Of-Plane Bending | 2.20617 | 2.34193 |
| vsn der Waals | 48.70837 | 48.76756 |
| Electrostatic | 1.42447 | 1.91772 |
| Total | 132.61700 | 124.42933 |
Spectroscopic Simulation using Quantum Mechanics
Using Gaussian an Optmisation and Frequency calculation[4] was carried out on Molecule 17 in order to establish it's NMR Spectrum. The calculated and literature data has been tabulated in Tables 5 & 6, and a link to the calculated spectra for both 1H and 13C are attached to the tables.
| Computed δ (ppm) | Integration | Literature[5] δ (ppm) | Integration |
|---|---|---|---|
| 5.21 | 1 | 4.84 | 1 |
| 3.04 | 1 | 3.40-3.10 | 4 |
| 2.93 | 1 | 2.99 | 1 |
| 2.82 | 3 | 2.80-1.35 | 14 |
| 2.76 | 1 | 1.38 | 3 |
| 2.58 | 1 | 1.25 | 3 |
| 2.52 | 1 | 1.10 | 3 |
| 2.30 | 1 | 1.00-0.8 | 1 |
| 2.11 | 1 | ||
| 1.87 | 3 | ||
| 1.63 | 1 | ||
| 1.47 | 3 | ||
| 1.39 | 1 | ||
| 1.30 | 2 | ||
| 1.12 | 1 | ||
| 0.88 | 1 | ||
| 0.81 | 1 | ||
| 0.74 | 1 | ||
| 0.57 | 2 | ||
| 0.49 | 1 | ||
| 0.30 | 2 |
| Computed δ (ppm) | Literature[5] δ (ppm) |
|---|---|
| 211.99 | 218.79 |
| 160.64 | 144.63 |
| 118.92 | 125.33 |
| 94.75 | 72.88 |
| 70.06 | 56.19 |
| 64.04 | 52.52 |
| 55.82 | 48.50 |
| 49.94 | 46.80 |
| 48.34 | 45.76 |
| 45.67 | 39.80 |
| 43.62 | 38.81 |
| 42.73 | 35.85 |
| 40.02 | 32.66 |
| 34.89 | 28.79 |
| 34.36 | 28.29 |
| 33.94 | 26.88 |
| 27.80 | 25.66 |
| 24.50 | 23.86 |
| 23.69 | 20.96 |
| 21.70 | 18.71 |
It is important to note in the calculated NMR that where the integration is 3 this does not mean a triplet exists, rather that the 3 H's are in very similar chemical environments so their chemical shifts are so close that Gaussian has grouped them together under one signal. The methyl hydrogens are identifiable and comparable with the literature values being at the lower end of the spectrum (cal: 0.30--1.39ppm, lit: 1.10-1.38ppm) although the range of values is very different. The two are difficult to compare due to the frequency at which the NMRs have been carried out at. The literature values are determined at 400MHz whereas the computed spectra it as an undetermined frequency. The discrepancy between these can be part of the reason for slightly different δ values for the same Hydrogens/carbons.
As the literature findings do not specify which shift relates to which atom it is difficult to determine if the spectra has been correctly assigned. As the two have similar ranges and follow the same general pattern it can be concluded that Molecule 17 was made experimentally. The lack of multiplets etc causes the computed NMR data to appear very different to the literature values but on inspection it is seen that the 14H multiplet is merely split out into singlets in the computed version as there is no coupling observed. The literature includes through space interactions which have not been calculated so the extensive coupling seen in the literature is not reflected in the computed data. As there is a general overlap of the two sets of data computing the coupling interactions is not necessary but if for example there was more discrepancy between the two spectra it could be employed to evaluate the literature assignments.
Part 2
Catalysts
On the left the crystal structure of the Shi catalyst can be seen. Using Conquest-Mercury the C-O bond lengths of the anomeric centres were measured with interesting results. There were slight differences in the bond lengths both with the anomeric ring and between the two of them. These lengths have been displayed in Figure 2 along with an optimised 2,2-Dimethyl-1,3-dioxolan to compare them to. Where both carbons are attached to the molecule it shall be referenced as A2 and where only on carbon is attached shall be referenced as A1.
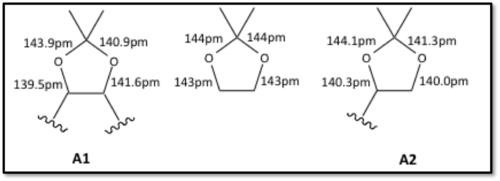
In A2 the ring structure is distorted due to it being fused to the central 6-membered ring leading to a lack of flexibility. The C-O bond closest to the carbonyl has a shorter bond length potentially down to favourable interactions of the two oxygen molecules. In A1 the C-O where the carbon is connected to the ring is significantly shorter than expected due to the close proximity of the cyclic oxygen on the other side of it causing the bond to contract. To compensate for this added strain on the 5-membered ring the adjacent C-O bond is close to the expected value.

To the left is the crystal structure of the Jacobsen catalyst found using Conquest-Mercury. It has an interesting structure as the tBu groups on the outer rings appear to be in close proximity and unfavourable steric interactions are expected between such bulky groups. When measured however all of the H-H bond distances were greater than 2Â indicating attractive interactions between them as below 2.1Â the interactions become repulsive. The C-C bond distances are all above 3.6Â and are tabulated in Table 7. The planar ring structure of the molecule causes the tBu groups to be far enough apart to not cause steric repulsion. This will aid the selectivity of the catalyst as the steric bulk of having tBu groups on the aryl rings results in a very crowded Mn centre. This is essential for the Jacobsen catalyst to be able to react selectivity to give >90% ee when epoxidising an alkene[6].
| Bond | Length (Â) |
|---|---|
| C21-C33 | 5.484 |
| C23-C35 | 3.696 |
| C24-C34 | 7.606 |
| C22-C36 | 3.861 |
NMR
Styrene Oxide
The computed 1H and 13C NMR and the related literature findings for Styrene oxide have been tabulated in Tables 8 & 9 below.
| Calculated[7] δ (ppm) | Literature[8] δ (ppm) |
|---|---|
| 2.5 | 2.79 |
| 3.1 | 3.13 |
| 3.7 | 3.85 |
| 7.3 | 7.33-7.37 |
| 7.5 | - |
| Calculated[7] δ (ppm) | Literature [8] δ (ppm) |
|---|---|
| 53.5 | 51.3 |
| 54.1 | 52.4 |
| 118.3 | 125.5 |
| 123.0 | 128.2 |
| 123.4 | 128.8 |
| 124.1 | 137.6 |
| 135.1 | - |
Due to the sensitivity that the shifts can be reported to using computational methods, where in experimental cases the atoms are seen as being in the same environment they can sometimes be recorded individually. However on the whole a general match can be seen between the computational and literature assignments leading to confidence in the optimized Styrene Epoxide.
Another way to check the integrity of what has been predicted is to use the Optical Rotation Dispersion (ORD) and compare the computational values with the literature. For Styrene oxide the ORD(λ=589nm) was -30.15 and ORD(λ=365nm) was -94.17[9]. These are in line with literature values found for λ=589 at -33.3[10]. These values being close is another indicator that the predicated product matches that widely found in the literature.
trans-Stilbene Oxide
The computed 1H and 13C NMR and the related literature findings for trans-Stilbene oxide have been tabulated in Tables 10 & 11 below.
| Calculated[11] δ (ppm) | Literature [12] δ (ppm) |
|---|---|
| 3.5 | 3.95 |
| 7.5 | 7.40-7.55 |
| 7.6 | - |
| Calculated[11] δ (ppm) | Literature [12] δ (ppm) |
|---|---|
| 66.4 | 62.7 |
| 118.3 | 125.5 |
| 123.1 | 128.2 |
| 123.2 | 128.5 |
| 123.5 | 137.1 |
| 124.2 | - |
| 134.1 | - |
As with Styrene Oxide there are issues surrounding the quantity of peaks collected by NMR analysis but again this can be put down to the sensitivity of the computational method vs the experimental machines. A general fit is seen again with this data and shifts are in the expected range of ppm for the molecule.
The ORD for trans-Stilbene Oxide have also been calculated[13] and found to be ORD(λ=589) 298.28 and ORD(λ=365) 1255.20. Although there is a larger difference between these values and the literature of ORD(λ=589) 319.8 [14] in comparison to that of Styrene oxide it is still within an acceptable range to confirm that the epoxide being used computationally matches the one found in literature.
Transition State Analysis
Shi Epoxidation of Styrene
| (R) Styrene Jmol | (S) Styrene Jmol | ||||||
|---|---|---|---|---|---|---|---|
|
|
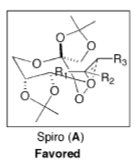
The lowest energy (R) and (S) Styrene epoxides Jmols are shown in Table 12. The energies of all of the transition states and the resulting K values can be found in Table 13. From the K value calculated the enantiomeric excess (ee) has been calculated as 23.84% in favour of the (S) product which is complimentary of the literature values which range from 11-26% ee for styrene and its derivativesCite error: Invalid parameter in <ref> tag. At a first look this seems extremely low however it is the lack of substitution on the alkene that leads to such poor selectivity in comparison to tri-substituted alkenes where the selectivity with the Shi catalyst is often above 90%. Due to the importance of sterics in the spiro transition state when using the Shi catalyst, seen in Figure 3, the lack of substituent on the terminal alkene in styrene results in poor selectivity for either S or R[15]. In order to increase the ee for styrene epoxidation a modified version of the shi catalyst can be used which includes a carbocyclic oxazolidinione containing keotine. For other terminal alkenes it is suggested that a modified version of the Shi catalysed to be used which doesn't rely upon steric effects for selectivity[15]. There have been other studies into the epoxidation of terminal alkenes and how to increase the selectivity of the reaction to yield great ee's but often these are not based on Fructose or ketone containing compounds[16].
The (S) epoxide being more stable indicates more favourable interactions between the methyl groups of the catalyst with styrene indicated by the shorted through space distances; (S) TS: 3.78A, (R) TS: 3.92A. Both values indicative attractive interactions (check with NCI of R) but more so for the (S) enantiomer. Interestingly in the (S) TS Styrene retains the majority of its planarity whereas in (R) the terminal alkene is bent up towards the catalyst which could be seen to contribute to the (S) being the more favourable TS for epoxidation as Styrene has not been distorted.
| Shi | ||||||
|---|---|---|---|---|---|---|
| TS | Electronic and Thermal Energies | ΔG | lnK | K | ||
| R | S | Hartree | j/mol | |||
| 1 | -1303.730703 | -1303.733828 | 0.003125 | 8204.686312 | -3.309917336 | 0.036519192 |
| 2 | -1303.730238 | -1303.724178 | -0.006060 | -15910.5277 | 6.418591699 | 613.1390213 |
| 3 | -1303.736813 | -1303.727673 | -0.009140 | -23997.06653 | 9.680846225 | 16008.0376 |
| 4 | -1303.738044 | -1303.738503 | 0.000459 | 1205.104326 | -0.486160658 | 0.614982997! |
| ee Calculations | |||
|---|---|---|---|
| R | S | ee | ee% |
| 0.380798435 | 0.619201565 | 0.238403131 | 23.84 |
Jacobsen Epoxidation of cis-B-methyl Styrene
| (S,R) Styrene Jmol | (R,S) Styrene Jmol | ||||||
|---|---|---|---|---|---|---|---|
|
|
The two Lowest energy TS Jmol's can be seen above in Table 15 and all the energies of the potential transition states can be seen in Table 16 below. As before, the enantiomeric excess was calculated by taking the difference in the Free Energies in order to find K and then using this to get the relative ratios of products. In this case an ee of 99.98% was calculated in favour of the (S,R) product which in the literature has an ee of 92% [17]. The high selectivity of this catalyst with cis-B-methyl styrene is put down to the alkene only being able to approach side on from one direction as seen in Figure 4.
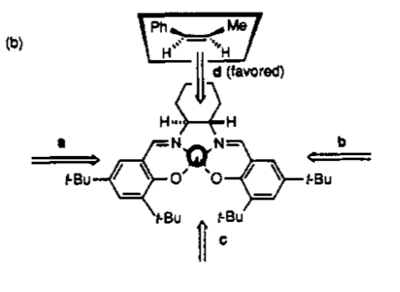
By examining both the (S,R) and (R,S) transition states the idea proposed that the face selectivity is attributed to the position of the phenyl group in relation to the axial hydrogen on the bridge[17] holds true. In the (R,S) TS this through space distance is 4.08A where as in the (S,R) TS it is larger at 4.67A indicating that the interaction of these two parts is repulsive causing the (S,R) transition state to be the more stable one.
| Jacobsen | ||||||
|---|---|---|---|---|---|---|
| TS | Electronic and Thermal Energies | ΔG | lnK | K | ||
| S,R | R,S | Hartree | j/mol | |||
| 1 | -3383.259559 | -3383.251060 | 0.008499 | 22314.12127 | -9.001915981 | 0.000123174 |
| 2 | -3383.253442 | -3383.250270 | 0.003172 | 8328.084795 | -3.359698493 | 0.034745733! |
| ee Calculations | |||
|---|---|---|---|
| S,R | R,S | ee | ee % |
| 0.999876842 | 0.000123158 | 0.999753683 | 99.98 |
NCI & QTAIM
The 4th (R)-Styrene epoxide transition state has been the focus of NCI and QTAIM analysis. From these the interactions between styrene and the Shi catalyst can be reviewed and maybe help to determine why this is not the lowest energy transition state and hence why (R) Styrene epoxide is the minor product.
Orbital |
As can be seen in the Jmol there is no blue surface between the catalyst and Styrene showing that there isn't a large amount of strongly attractive forces between the two. There is a small patch of blue on the ring which is between the new -C-O- bond. The attractive interactions between the methyl groups of the catalyst with Styrene can clearly be seen by the green surface between them has slight yellow tones indicating that this dispersion is not entirely attractive.
In comparison with the QTAIM where there are strong green surfaces where a BCPs are found, showing the validity of the two calculations. However the lack of co-ordinates available for the QTAIM make it difficult to quantitatively compare the two. Qualitatively though H-Bonds can be seen between the Oxygen and Hyrdrogens on the catalyst.
The main area of dispersion is between the alkene and A1 anomeric centre leading to the spiro transition state as expected[15]. The lack of substitution on the double bond results in little interaction at the terminal end of the alkene with the catalyst, perhaps indicative of the poor selectivity for this reaction; if the catalyst could interact with an area of steric bulk it would be more selective towards one enantiomer due to the attractive forces between the two molecules.
All of the non-bond BCPs in the QTAIM are about central indicating a balanced contribution from both atoms involved, and the majority of these are H-bonds so an equal contribution is expected. Interestingly the Shi Catalyst has a couple of intr-amolecular BCPs which could explain the stability of this catalyst and its selectivity. The terminal Hydrogen has two non-bonding BCPs to the catalysis which demonstrate the catalyst's selectivity mechanism being based upon steric interactions. If these hydrogens were replaced with larger groups than stronger interactions would be seen here.
Other Candidates
Chrysene is another molecule that could be investigated and is readily available from Sigma Aldrich[18] and has an ORD of 9. This would be an interesting investigation due to the conjugation of the system and selectivity required to epoxidise the double bond. The Jacobsen catalyst would be the most sensible choice due to its selective use for cis-alkenes although a modified version may have to be used due to the size of the molecule. More selectivity may be required due to the quantity of bonds available to react and this may also require a modified version of the Jacobsen catalyst or one based on similar foundations.
References
- ↑ 1.0 1.1 QTAIM-DI-VISAB computational study on the Diels-Alder reaction of cyclopentadiene - On the nature of the so-called secondary orbital interactions, N. Werstiuk, W. Sokol DOI:10.1139/v08-070
- ↑ 2.0 2.1 The first thermally-induced retro-oxy-cope rearrangement Steven W. Elmore1, Leo A. Paquette DOI:10.1016/S0040-4039(00)92617-0
- ↑ Evaluation and prediction of the stability of bridgehead olefins Wilhelm F. Maier , Paul Von Rague Schleyer DOI:10.1021/ja00398a003
- ↑ Olivia Ashton D-Space DOI:10042/26174
- ↑ 5.0 5.1 [3.3] Sigmatropy within 1-vinyl-2-alkenyl-7,7-dimethyl-exo-norbornan-2-ols. The first atropselective oxyanionic Cope rearrangement Leo A. Paquette , Neil A. Pegg , Dana Toops ,George D. Maynard , Robin D. RogersDOI:10.1021/ja00157a043
- ↑ The Mechanistic Basis for Electronic Effects on Enantioselectivity in the (salen)Mn(III)-Catalyzed Epoxidation Reaction Michael Palucki, Nathaniel S. Finney, Paul J. Pospisil, Mehmet L. Gu1ler, Toyohisa Ishida, and Eric N. Jacobsen DOI:10.1021/ja973468j
- ↑ 7.0 7.1 Olivia Ashton D-Space DOI:10042/26247
- ↑ 8.0 8.1 ENVIROCAT (K10-MX)–CATALYZED REGIOSELECTIVE TRANSFORMATION OF ALKENES INTO IODOHYDRINS AND b-IODO ETHERS AND FURTHER CONVERSION OF IODOHYDRINS TO EPOXIDES USING Al2O3-Na2CO3 UNDER MWI Shallu, M. L. Sharma, and Jasvinder Singh DOI:10.1080/00397911.2010.539755
- ↑ Olivia Ashton D-Space DOI:10042/26246
- ↑ Stereochemistry and Mechanism of the Photochemical and Thermal Insertion of Oxygen into the Carbon-Cobalt Bond of Alkyl(pyridine)cobaloximes Frederick R. Jensen and Ronald C. Kiskis DOI:10.1021/ja00853a029
- ↑ 11.0 11.1 Olivia Ashton D-Space DOI:10042/26248
- ↑ 12.0 12.1 Sequential- and tandem-oxidation–epoxidation reactions employing guanidine bases, David J. Phillips, Joseline L. Kean, Andrew E. Graham DOI:10.1016/j.tet.2013.05.036
- ↑ Olivia Ashton D-Space DOI:10042/26397
- ↑ A Diacetate Ketone-Catalyzed Asymmetric Epoxidation of Olefins Bin Wang, Xin-Yan Wu, O. Andrea Wong, Brian Nettles, Mei-Xin Zhao, Dajun Chen, and Yian Shi DOI:10.1021/jo900330n
- ↑ 15.0 15.1 15.2 15.3 Exploring structural effects of ketone catalysts on asymmetric epoxidation of olefins Zackary Crane, David Goeddel, Yonghong Gan,Yian Shi DOI:10.1016/j.tet.2005.03.123.
- ↑ An Efficient Catalyst for Asymmetric Epoxidation of Terminal Olefins James P. Collman, Zhong Wang, Andrei Straumanis, and Me ́lanie Quelquejeu DOI:10.1021/ja9818699
- ↑ 17.0 17.1 17.2 Highly Enantioselective Epoxidation Catalysts Derived from 1,2-Diaminocy~lohexaneEric N. Jacobsen, Wei Zhang, Alexander R. Muci, James R. Ecker, and Li Deng DOI:10.1021/ja00018a068
- ↑ Chrysene Sigma Aldrich http://www.sigmaaldrich.com/catalog/product/aldrich/245186?lang=en®ion=GB
