Rep:Mod:OJA1811
Computational Labs: Module 1
Optimization
In this exercise BH3, BBr3 and GaBr3 have been optimized with varying basis sets and pseudo potentials as appropriate. For each optimization a Summary Table and Item table is provided.
BH3 3-21G
For the first optimization of BH3 the basis set 3-21G was used. This basis set is not very accurate and so the calculations only took 19 seconds to complete. A link to the log file can be found below. Media:OJABH3OPTIMISATION.LOG
Summary Table
| BH3 Optimisation Summary | |
|---|---|
| File Name | OJABH3OPTIMISATION |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.46226338 a.u. |
| RMS Gradient Norm | 0.00020672 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Calculation Duration | 19 seconds |
Optimisation Calculations
Item Value Threshold Converged? Maximum Force 0.000413 0.000450 YES RMS Force 0.000271 0.000300 YES Maximum Displacement 0.001610 0.001800 YES RMS Displacement 0.001054 0.001200 YES Predicted change in Energy=-1.071764D-06 Optimization completed. -- Stationary point found.
Bond Information
| BH3 Bond Information | |
|---|---|
| B-H Bond Length | 1.19Å |
| H-B-H Bond Angle | 120.0° |
Total Energy From the optimization using a 3-21G basis set was calculated at -26.46226338 a.u.
BH3 6-31G(d,p)
In this case BH3 has been optimized again but with a more accurate basis set in order to obtain more reliable information on the bonds in the molecule. Media:OJABH3OPTIMISATION631GDP.LOG
Summary Table
| BH3 Optimisation Summary | |
|---|---|
| File Name | OJABH3OPTIMISATION631Gdp |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61532252 a.u. |
| RMS Gradient Norm | 0.00020672 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Calculation Duration | 1 minute 40 seconds |
Optimisation Calculations
Item Value Threshold Converged? Maximum Force 0.000433 0.000450 YES RMS Force 0.000284 0.000300 YES Maximum Displacement 0.001702 0.001800 YES RMS Displacement 0.001114 0.001200 YES Predicted change in Energy=-1.189019D-06 Optimization completed. -- Stationary point found.
Bond Information
| BH3 Bond Information | |
|---|---|
| B-H Bond Length | 1.19Å |
| H-B-H Bond Angle | 120.0° |
Total Energy From the optimization using a 6-31G(d,p) basis set was calculated at -26.61532252 a.u.
GaBr3
Summary Table
| GaBr3 Optimisation Summary | |
|---|---|
| File Name | OJA_GABR3_OPT_OUTPUT |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -41.70082783 a.u. |
| RMS Gradient Norm | 0.0000001620672 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Calculation Duration | 23 seconds |
Optimisation Calculations
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000002 0.001200 YES Predicted change in Energy=-1.282678D-12 Optimization completed. -- Stationary point found.
Bond Information
| GaBr3 Bond Information | ||
|---|---|---|
| Ga-Br Bond Length | Br-Ga-Br Bond Angle | |
| Calculated | 2.35 Å | 120.0° |
| Literature [1] | 2.24 Å | 123.1° |
BBr3
Summary Table
| BBr3 Optimisation Summary | |
|---|---|
| File Name | OJA_BBR3_OPT__GEN_OUTPUT_2 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | GEN |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -64.43645296 a.u. |
| RMS Gradient Norm | 0.00000382 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Calculation Duration | 32.7 seconds |
Optimisation Calculations
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES Predicted change in Energy=-4.026784D-10 Optimization completed. -- Stationary point found.
Bond Information
| BBr3 Bond Information | |
|---|---|
| B-Br Bond Length | 1.93 Å |
| Br-B-Br Bond Angle | 120.0° |
Analysis
| Molecule | Calculated Bond Lengths | Predicted Bond Lengths [2] [3] |
|---|---|---|
| BH3 | 1.19 Å | 1.16 Å |
| BBr3 | 1.93 Å | 1.93 Å |
| GaBr3 | 2.35 Å | 2.35 Å |
The calculated bond lengths are in line with those predicted using the covalent radii of the atoms. There is a noticeable difference in BBr3 however, and this may be due to the pi back donation from bromine into the empty p orbital of the boron causing the actual bond length to be shorter as there is a weak element of a double bond. This cannot occur with hydrogen as it has no other electrons to share, however as it is much smaller than bromine its orbitals are not diffuse so it can sit closer to the boron centre. There is a greater difference in electronegativity of B-Br than B-H indicating that B-Br will have a more polarized covalent bond than B-H. This in turn could result in the electron density not being held tightly between the two atoms so the bond is not as restricted, hence a longer bond in BBr3.
Gallium is significantly bigger than Boron so a longer bond is expected for the obvious reason of the valence orbitals in gallium being far more diffuse than those of Boron. Despite the same number of valence electrons, Boron is a non metal and forms a covalent bond with Bromine and hydrogen where as gallium is a metal so its bond with Bromine is likely to be predominantly ionic. There will be a certain degree of repulsion between the two atoms and the longer bond length indicates that there will be a greater degree of repulsion between Ga and Br than B and Br.
Once GaBr3 had been optimized Gaussview did not show the bonds between the atoms. This doesn't mean that the bond does not exist, rather that it exceeds Gaussview's predefined value of what a bond is. More loosely a bond can be described as what happens when two elements are effectively stuck to a shared electron density. This density may be spread unevenly between them, but the attraction to it is enough to keep the nuclei together and in the absence of other molecules, keeps the two elements together. On a basic level a bond can be seen to be the result of two individual atoms coming together and acting as a single body due to a force between them.
References
- ↑ http://www.sciencedirect.com/science/article/pii/S0022286097004201# Gallium tribromide: molecular geometry of monomer and dimer from gas-phase electron diffraction Balazs RCffya, MAria Kolonits, Magdolna Hargittaibs
- ↑ CRC Handbook of Chemistry and Physics, CRC Press, 92nd Edition, 2011.
- ↑ G. W. C. Kaye and T. H. Laby Tables of Physical and Chemical Constants, Longman, 16th Edition, 1995.
Frequency
As both the Ground state and the Transition state of a molecule have a 0 gradient along the potential energy curve it is important to determine whether the optimized geometries are ground states. In order to do this vibrational analysis is carried out on the molecules and the frequencies analysed. By doing this a second derivative is taken of the potential surface and if the molecule is in its ground state then all the frequencies calculated should be positive. This analysis has been carried out on BH3 and GaBr3. These calculations are also useful as the IR spectrum can be extracted and analysed, which can give important information about the molecules.
When carrying out a frequency analysis the basis set must be kept consistent with that of the optimization otherwise the system will not be appropriately optimized for the calculations. There are two types of "Low Frequencies" which have been included for both molecules. The first ones which are very small numbers are the -6 frequencies of the 3N-6 modes a molecule has and indicate the movement of the center of mass of the molecule. These are the small numbers which are very close to 0, indicating that the calculation is valid. The second set are the "real" frequencies of the molecule, and appear in the IR Spectrum.
For each frequency calculations a Summary table, "Low frequency" and Frequency table has been included. A comparison of the results can be found in the Analysis section.
BH3
The molecule of BH3 that was optimised with a 6-31G(d,p) basis set was used in order to be as accurate as possible. A link to the full calculation file can be found here: Media:OJA_BH3_FREQ.LOG
Summary Table
| BH3 Frequency Summary | |
|---|---|
| File Name | OJA_BH3_FREQ |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61532363 a.u. |
| RMS Gradient Norm | 0.00000237 a.u. |
| Imaginary Freq | 0 |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Time | 47 seconds |
Frequency Calculations
Low frequencies --- -0.9033 -0.7343 -0.0054 6.7375 12.2491 12.2824 Low frequencies --- 1163.0003 1213.1853 1213.1880
Vibrations
From the frequency calculations the IR-active vibrations can be deduced. A summary table below shows the movement of the molecule and its symmetry for each wavelength. By clicking on the thumbnail an animation will begin.
Spectrum
The IR spectrum of BH3 can be seen below. Despite the fact that there are 6 separate vibrations there are only 3 peaks in the spectrum. There is no peak at 2582.26 due to its 0 intensity as the motion causes no change in dipole moment of the moelcule so is not IR active. For the E' symmetry motions the change in dipole moment will be very similar and as their intensities are so close no separation of the peaks can be seen.

GaBr3
The complete vibrational analysis calculation file for GaBr3 can be found here: DOI:10042/25267
Summary Table
| GaBr3 Frequency Summary | |
|---|---|
| File Name | OJA_GABR3_FREQ_OUTPUT |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -41.70082783 a.u. |
| RMS Gradient Norm | 0.00000011 a.u. |
| Imaginary Freq | 0 |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Time | 14 seconds |
Frequency Calculations
Low frequencies --- -0.5252 -0.5247 -0.0024 -0.0010 0.0235 1.2010 Low frequencies --- 76.3744 76.3753 99.6982
Vibrations
The frequencies and their intensities of the vibrations of GaBr3 are tabulated below.
| No. | Frequency (cm-1) | Intensity |
|---|---|---|
| 1 | 76 | 3 |
| 2 | 76 | 3 |
| 3 | 100 | 9 |
| 4 | 197 | 0 |
| 5 | 316 | 57 |
| 6 | 316 | 57 |
Spectrum
The calculated IR spectrum of GaBr3 can be found below. Again there are not 6 distinct peaks seen on this spectrum due to certain frequencies being almost identical and the lack of change in dipole moment leading to 0 intensity as it the vibration is not IR active.

Analysis
| BH3 | GaBr3 | ||||
|---|---|---|---|---|---|
| Frequency (cm-1) | Intensity | Symmetry | Frequency (cm-1) | Intensity | Symmetry |
| 1163 | 93 | A2" | 76 | 3 | E' |
| 1213 | 14 | E' | 76 | 3 | E' |
| 1213 | 14 | E' | 100 | 9 | A2" |
| 2582 | 0 | A1' | 197 | 0 | A1' |
| 2715 | 126 | E' | 316 | 57 | E' |
| 2715 | 126 | E' | 316 | 57 | E' |
Overall GaBr3 frequencies are lower than BH3 frequencies. This is due to Energy (E) being inversely proportional to wavelength (λ), and the wave number (v) being the reciprocal of wavelength meaning that E ∝ v. Complimenting the bond length information derived from the earlier optimization calculations, the IR proves that BH3 bonds are far stronger than GaBr3 bonds. This is related to their relative bond lengths with B-H bonds being considerably shorter than Ga-Br bonds. Shorter bond lengths result in stronger bonds as the electrons are held closely to both nuclei so they experience greater attraction from the nuclei resulting in more energy being needed to overcome this attraction. In GaBr3 bonds the electrons are more diffuse from the two nuclei so less energy is required to break the bond.
Despite the molecules having the same geometry there has been a reordering of modes which can be clearly seen when comparing the IR frequencies. The two E' bond bending modes are lower in energy than the A2" bond bend in GaBr3 which is the reverse for BH3. In both cases the A2" and E' modes and the A1' and E' modes lie close together but are separating by a significant amount. This is because the lower frequencies are bond bending movements whereas the high frequencies are bond stretching. Bending bonds requires less energy than stretching as it is the angles between the atoms which are distorted whereas bond stretching involves the extension and compression of bonds.
Molecular Orbitals
To determine the molecular orbitals of a molecule the promised file is taken and an energy calculation is carried out. These orbitals can then be compared to the approximated ones too see how accurate a qualitative Molecular Orbital diagram is. In this example the Molecular orbitals of BH3 have been calculated and compared to the ones predicted by employing MO theory.
BH3
The complete energy calculation file for BH3 can be found here: DOI:10042/25323
Diagram
Below the MO diagram for BH3 is shown, with images of the calculated MOs being displayed next to the predicted ones. The biggest difference is the consistency between filled and unfilled orbitals, depending on the LCAOs the green can be either filled or unfilled. The general form of the calculated orbitals matches the predicted ones, and identifying which orbitals correlated wasn't difficult indicating that using MO theory is a useful qualitative method in the absence of computational analysis. However if accurate information or further calculations were to be conducted it would be advisable to use the computational method in order to avoid an increase in error. An interesting point to note would be the order of the 3a'1and 2e' orbitals. In the predicted model there is ambiguity about the ordering of these two orbitals however the calculations conducted confirm the order of these two orbitals with 3a'1 is placed below the 2e' as shown below.

NBO Analysis
In order to evaluate the Natural bond orbitals an optimization and vibrational analysis of the molecule must first be carried out in order to establish it is in it's ground state configuration. Then the charge distribution of the molecule can be reviewed. For this exercise NH3 has been used.
NH3
Optimisation Calculation: Media:OJA_NH3_OPT_631GDP_5.LOG
Optimisation Summary Table
| NH3 Optimisation Summary | |
|---|---|
| File Name | OJA_NH3_OPT_631GDP_5 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -56.55776863 a.u. |
| RMS Gradient Norm | 0.00000279 a.u. |
| Imaginary Freq | |
| Dipole Moment | 1.8464 Debye |
| Point Group | C3V |
| Calculation Duration | 1 minute 15 seconds |
Calculations
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000010 0.001800 YES RMS Displacement 0.000007 0.001200 YES Predicted change in Energy=-7.830149D-11 Optimization completed. -- Stationary point found.
Frequency Calculation: Media:OJA_NH3_FREQ_5.LOG
Frequency Summary
| NH3 Frequency Summary | |
|---|---|
| File Name | OJA_NH3_FREQ_5 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -56.55776856 a.u. |
| RMS Gradient Norm | 0.00000279 a.u. |
| Imaginary Freq | 0 |
| Dipole Moment | 1.8464 Debye |
| Point Group | C3 |
| Time | 41 seconds |
Frequency Calculations
Low frequencies --- -11.6527 -11.6490 -0.0045 0.0333 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
As discussed in the earlier Frequency section, the first 6 low frequencies should be close to 0 to prove the validity of the calculation. However in this calculation one of the frequencies is unusually high, at ~25cm-1, giving slight cause for concern. On review of the .log file related to this it was found that all forces were converged and very close to 0 therefore the calculation was successful. In order to minimize this frequency a more accurate basis set or method would be required but for the purpose of this exercise it is not appropriate to investigate that here.
Molecualr Orbitals
Energy Calculation: DOI:10042/25321
Charge Distribution

Using NBO analysis the charge distribution of NH3 can be viewed through a colour coded representaiton. Green indicates poisitive charge and red indicates negative charge; as expcected the central nitrgoen is hgihly negatively charged. The charge limits in the case are from -1.125 to +1.125. Below the actual charges of the atoms has been tabulated. Hydrogen is very slightly positively charged, hence its green colouring in the adjacent image.
| Atom | Charge |
|---|---|
| N | -1.125 |
| H | +0.375 |
Reaction Energies
Accumulating the skills learned from the above exercises in optimization, vibrational analysis, molecular orbitals and NBO Analysis, the reaction energies for the formation of NH3BH3 can be calculated. By using the optimization energies the dative N-B bond energy can be found and then with comparison to the separate NH3 and BH3 energies the association/dissociation bond energy can be found.
NH3BH3
Optimisation: Media:OJA_NH3BH3_OPT_631GDP.LOG
Summary Table
| NH3BH3 Optimisation Summary | |
|---|---|
| File Name | OJA_NH3BH3_OPT_631GDP |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -83.22469007 a.u. |
| RMS Gradient Norm | 0.00006839 a.u. |
| Imaginary Freq | |
| Dipole Moment | 5.5653 Debye |
| Point Group | C1 |
| Calculation Duration | 3 minutes 7 seconds |
Calculations
Item Value Threshold Converged? Maximum Force 0.000139 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000771 0.001800 YES RMS Displacement 0.000338 0.001200 YES Predicted change in Energy=-2.028055D-07 Optimization completed. -- Stationary point found.
Frequency: Media:OJA_NH3BH3_FREQ.LOG
Summary Table
| NH3BH3 Frequency Summary | |
|---|---|
| File Name | OJA_NH3BH3_FREQ |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -83.22469000 a.u. |
| RMS Gradient Norm | 0.00006830 a.u. |
| Imaginary Freq | 0 |
| Dipole Moment | 5.5653 Debye |
| Point Group | C1 |
| Time | 1 minute 22 seconds |
Calculations
Low frequencies --- -0.0015 -0.0010 -0.0007 18.9885 23.6408 42.9617 Low frequencies --- 266.5827 632.3776 639.4313
Dissociation Energy
| Molecule | Energy |
|---|---|
| NH3 | -56.56 a.u. |
| BH3 | -26.61 a.u. |
| NH3BH3 | -83.22 a.u. |
In order to find the bond energy and hence the dissociation energy of NH3BH3, the relative energies of the molecuels were taken from the calculaitons conducted and used in the following equation. Calculation ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22 - (-26.61 + -56.56) = -0.05a.u. = -0.05*2625.50 kj/mol = -131.275kj/mol
The bond dissociation energy of NH3BH3 has been determined to be -131.28 kjmol-1. In comparison with a literature value found at 30.7 kcal/mol (128.45kj/mol) [1] this method of determining bond dissociation energies can be taken to be successful.
References
Computational Labs: Module 2
D-Space Links
| Calculation | Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|---|
| Optimisation | DOI:10042/25676 | DOI:10042/25669 | DOI:10042/25667 | DOI:10042/25672 |
| Frequency | DOI:10042/25675 | DOI:10042/25666 | DOI:10042/25665 | DOI:10042/25671 |
| Energy | DOI:10042/25673 | DOI:10042/25668 | DOI:10042/25664 | DOI:10042/25670 |
Optimisation
Summary Table
| Optimization | Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|---|
| File Name | OJA_BENZ_OPT_631GDP_OUTPUT | OJA_BORAT_OPT_631GDP_OUTPUT | OJA_PYR_OPT_631GDP_OUTPUT | OJA_BORZ_OPT_631GDP_OUTPUT |
| File Type | .log | .log | .log | .log |
| Calculation Type | FOPT | FOPT | FOPT | FOPT |
| Calculation Method | RB3LYP | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 0 | -1 | 1 | 0 |
| Spin | Singlet | Singlet | Singlet | Singlet |
| E(RB3LYP) | -232.25820551 a.u. | -219.02052984 a.u. | -248.66807396 a.u. | -242.68458739 a.u. |
| RMS Gradient Norm | 0.00009550 a.u. | 0.00015822 a.u. | 0.00003896 a.u. | 0.00006610 a.u. |
| Imaginary Frequency | ||||
| Dipole Moment | 0.0001 Debye | 2.8465 Debye | 1.8727 Debye | 0.0002 Debye |
| Point Group | C1 | C1 | C1 | C1 |
| Time | 1 minute 13.8 seconds | 2 minutes 52.9 seconds | 2 minutes 54.5 seconds | 3 minutes 28.2 seconds |
Item Table
Benzene
Item Value Threshold Converged? Maximum Force 0.000212 0.000450 YES RMS Force 0.000085 0.000300 YES Maximum Displacement 0.000991 0.001800 YES RMS Displacement 0.000315 0.001200 YES Predicted change in Energy=-5.157444D-07 Optimization completed. -- Stationary point found.
Boratabenzene
Item Value Threshold Converged? Maximum Force 0.000159 0.000450 YES RMS Force 0.000069 0.000300 YES Maximum Displacement 0.000911 0.001800 YES RMS Displacement 0.000335 0.001200 YES Predicted change in Energy=-6.630189D-07 Optimization completed. -- Stationary point found.
Pyridinium
Item Value Threshold Converged? Maximum Force 0.000064 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.000702 0.001800 YES RMS Displacement 0.000174 0.001200 YES Predicted change in Energy=-6.897601D-08 Optimization completed. -- Stationary point found.
Borazine
Item Value Threshold Converged? Maximum Force 0.000093 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000338 0.001800 YES RMS Displacement 0.000096 0.001200 YES Predicted change in Energy=-1.027046D-07 Optimization completed. -- Stationary point found.
Frequency
Summary Table
| Optimization | Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|---|
| File Name | OJA_BENZ_FREQ_OUTPUT | OJA_BORAT_FREQ_OUTPUT | OJA_PYR_FREQ_OUTPUT | OJA_BORZ_FREQ_OUTPUT |
| File Type | .log | .log | .log | .log |
| Calculation Type | FREQ | FREQ | FREQ | FREQ |
| Calculation Method | RB3LYP | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 0 | -1 | 1 | 0 |
| Spin | Singlet | Singlet | Singlet | Singlet |
| E(RB3LYP) | -232.25820586 a.u. | -219.02052984 a.u. | -248.66807396 a.u. | -242.68458753 a.u. |
| RMS Gradient Norm | 0.00009558 a.u. | 0.00015830 a.u. | 0.00003894 a.u. | 0.00006600 a.u. |
| Imaginary Frequency | ||||
| Dipole Moment | 0.0001 Debye | 2.8465 Debye | 1.8727 Debye | 0.0002 Debye |
| Point Group | C1 | C1 | C1 | C1 |
| Time | 4 minutes 15.7 seconds | 5 minutes 0.4 seconds | 4 minutes 22.8 seconds | 5 minutes 7.5 seconds |
Low Frequencies
Benzene
Low frequencies --- -17.0537 -14.3774 -9.3919 -0.0009 -0.0006 0.0003 Low frequencies --- 413.7989 414.4772 620.8619
Boratabenzene
Low frequencies --- -13.1241 0.0008 0.0009 0.0009 15.0608 18.1734 Low frequencies --- 371.3450 404.2340 565.2523
Pyridinium
Low frequencies --- -7.2019 -0.0005 0.0002 0.0006 17.3427 18.5430 Low frequencies --- 392.4561 404.0619 620.4714
Borazine
Low frequencies --- -17.0998 -10.8330 -5.8654 0.0006 0.0013 0.0013 Low frequencies --- 288.8425 289.6877 404.1663
NBO Analysis
| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |
 |
 |
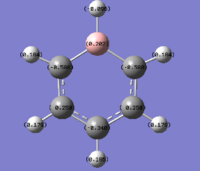 |
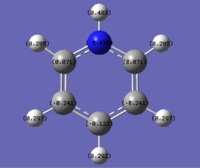 |
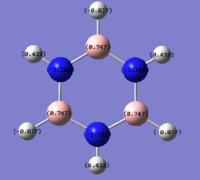 |
| Atom | Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|---|
| 1 | -0.239 | 0.202 | -0.476 | 0.747 |
| 2 | -0.239 | -0.588 | 0.071 | -1.102 |
| 3 | -0.239 | -0.250 | -0.241 | 0.747 |
| 4 | -0.239 | -0.340 | -0.122 | -1.102 |
| 5 | -0.239 | -0.250 | -0.241 | 0.747 |
| 6 | -0.239 | -0.588 | 0.071 | -1.102 |
| H-1 | 0.239 | -0.096 | 0.483 | -0.077 |
| H-2 | 0.239 | 0.184 | 0.285 | 0.432 |
| H-3 | 0.239 | 0.179 | 0.297 | -0.077 |
| H-4 | 0.239 | 0.186 | 0.292 | 0.432 |
| H-5 | 0.239 | 0.179 | 0.297 | -0.077 |
| H-6 | 0.239 | 0.184 | 0.285 | 0.432 |
| Atom | Electronegativity[1] [2] |
|---|---|
| C | 2.550 |
| H | 2.200 |
| N | 3.040 |
| B | 2.040 |
As can be seen from the images above, changing a single atom in the ring affects all other atoms in it. In benzene there is a uniform spread of electrons across the carbons as they are all the same. However in Boratabenzene the introduction of an electropositive boron causes the neighboring carbons to have an increase in electron density making them more negative and hence a bright red colour. The carbon opposite the boron shows higher electronegativities than those next to it, indicating at an uneven spread of electrons over the remaining carbon atoms.
The opposite of this can be seen in pyridinium. Here an electronegative atom has been introduced into the ring and that means that the electron density is drawn towards it, making the neighboring carbons far more electropositive than in benzene. This then leads to a build up of electron density on atoms 3 and 5 leaving atom 4 (opposite the nitrogen) more electropositive than carbon 4 in benzene.
The expected trend of distinct electropositive and electronegative sites is seen in Borazine. Due to the difference in electronegativities of boron and nitrogen, as seen in the table below, the electron density is held in a repeat pattern and is not smeared across the ring as seen in boratabenzene and pyridinium.
The changing electronegativities of the atoms in the ring has an effect on the charge distribution on the Hydrogen atoms. The more electronegative a ring atom is the more electron density that the nitrogen holds. This means that the phenomena seen on the ring atoms is the inverted pattern seen on the hydrogens.
References
MO Diagram of Benzene
Using the molecular orbitals of Benzene acquired through calculations an MO diagram with accompanying LCAOs has been constructed. The molecular orbitals calculated have been interpreted in order to form the LCAOS by using known information about the molecule, e.g. that the carbons are sp2 hybridized due to the planar nature of the molecule.
These MOs can be used to show that benzene is aromatic. For example the pi orbital arrangement of orbitals shows how the electrons can be delocalized around the ring as there are no breaks in the orbital. This can also be seen in the sigma orbitals as the orbitals can be seen to not be localized on to specific atoms but instead extend across the ring, indicating that the molecule is aromatic.
In order for a molecule to be aromatic it must hold 4n+2 electrons in it's pi orbitals. Benzene fulfills this brief and can be seen on the MO diagram. The 3 bonding pi orbitals hold two electrons each leading to 6 electrons in total. In this case n=1, and benzene fulfills the criteria to be aromatic.

MO comparison
Below is a comparison table of 3 of the molecular orbitals from Benzene, Boratabenzene, Pyridinium and Borazine.
By changing the certain atoms in a molecule the whole MO set will be affected, firstly in the relative energies of the orbital levels. A general trend can be seen throughout this set of molecules:
Boratabenzene < Benzene ~ Borazine < Pyridinium
This reflects the electronic structure of each of the complexes. Pyridinium carries a positive charge so is lacking in electrons and hence the whole molecule is higher in energy in comparison to the other three. Conversely Boratabenzene carries a negative charge making it lower in energy as it is electron rich. Benzene and borazine are neutral and are most similar in electron density distribution and so are close in energy.
The ordering of the MOs is also affected which includes where degenerate orbitals sit. As the symmetry of the LCAOS for the ring atoms will change due to the introduction of new atoms so will their energy levels, which results in the order being slightly different for each molecule. Below the ordering and energies of orbitals 7 to 27 can be seen, with degenerate orbitals highlighted in red.
Both pyridinium and Borazine have no degenerate molecular orbitals and this is due to a lack of symmetry in the molecule. When either N or B is replaces a Carbon in the benzene ring the orbital's eigen values change resulting in different energies for the orbitals.



















