Rep:Mod:OA010298
EX3 Molecules and Adduct
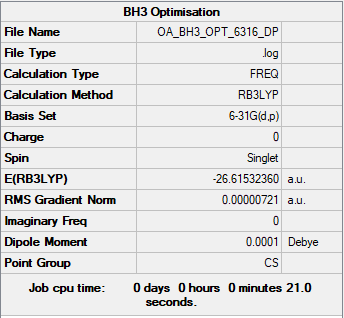
BH3
Borane
B3LYP/6-31G(d,p) level
Ng611 (talk) 01:46, 15 May 2019 (BST) Slight mistake here in that the point group of BH3 is actually C3V and not CS.
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000105 0.001800 YES RMS Displacement 0.000062 0.001200 YES
Frequency analysis log fileː Media:OLIVER_BH3_FREQ_OPT.LOG
Low frequencies --- -2.2126 -1.0751 -0.0055 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
Optimised BH3 Molecule |
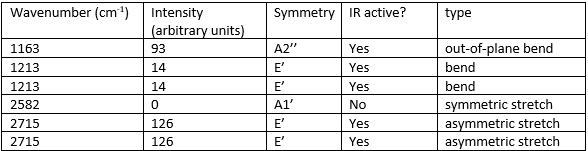
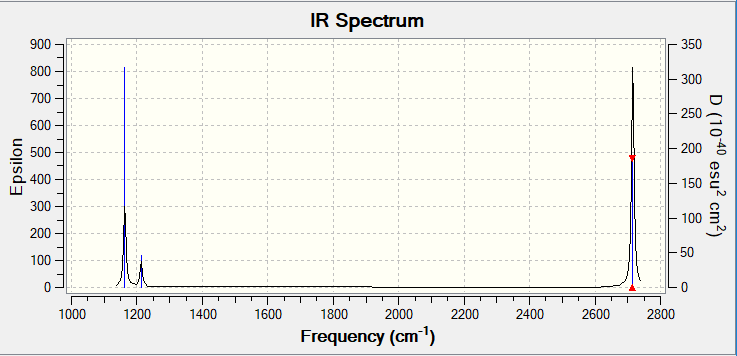
Vibrational spectrum for BH3ː
There are 6 different vibrations but only 3 peaks seen in the IR spectrum. One vibration isn't present since it doesn't induce a change in dipole moment and therefore isn't IR active (the A1' symmetric stretch). The 2 E' bends (at 1213 cm-1) are degenerate as are the 2 E' asymmetric stretches (at 2715 cm-1), this means their peaks overlap each other resulting in only one peak being seen per pair of vibrations.
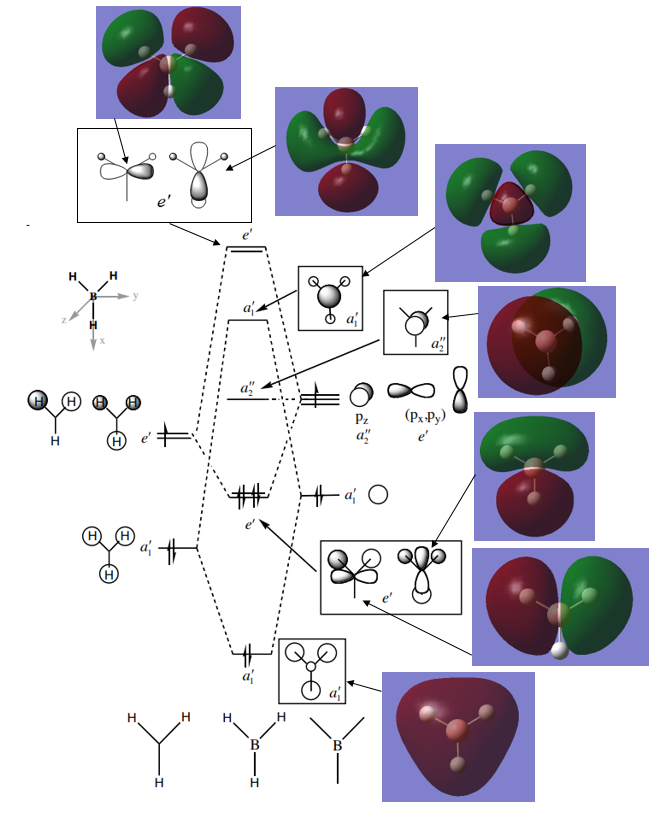
MO diagram for BH3ː
By comparing the LCAOs with the calculated MOs its clear that the LCAOs indicate well where nodes are as they both display the same nodes. The main difference between the 2 are the 'gaps' seen in the LCAOs between neighbouring orbitals of the same phase whereas the calculated MOs successfully merge these together, removing the 'gaps'. When combining AOs to form the LCAO diagrams the relative sizes of the orbitals being combined are simply assigned as large or small depending on whether the orbital in question is close or far in energy relative to the MO that is formed (when compared to the orbital it is being combined with). Since its very hard to accurately assign these sizing coefficients to orbitals there are some discrepancies between the LCAO diagrams and those from the calculations, notably the anti-bonding a1' orbital.
The LCAOs generally resemble very similar orbital arrangements to the calculated MOs and so are useful for indicating the rough shape of the MOs. They are accurate in this sense but to accurately determine the coefficients of the AOs used the calculated MO technique is more beneficial. ̇̈ Ng611 (talk) 01:44, 15 May 2019 (BST) Very good discussion, well done!
NH3
Ammonia
B3LYP/6-31G(d,p) level
NH3 optimistaion summary tableː
Item Value Threshold Converged? Maximum Force 0.000050 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.000404 0.001800 YES RMS Displacement 0.000180 0.001200 YES
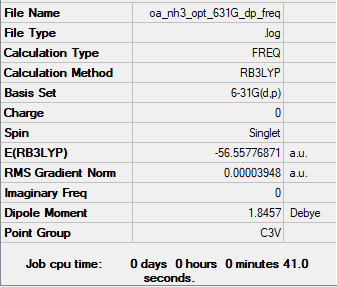
Frequency analysis log fileː Media:OA_NH3_OPT_631G_DP_REAL_FREQ.LOG
Low frequencies --- -30.5926 -30.5914 -0.0048 0.0121 0.0483 4.7247 Low frequencies --- 1088.6632 1694.0281 1694.0281
Optimised NH3 Molecule |
NH3BH3
NH3BH3 Adduct
B3LYP/6-31G(d,p) level
NH3BH3 optimistaion summary tableː
Item Value Threshold Converged? Maximum Force 0.000120 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000682 0.001800 YES RMS Displacement 0.000178 0.001200 YES
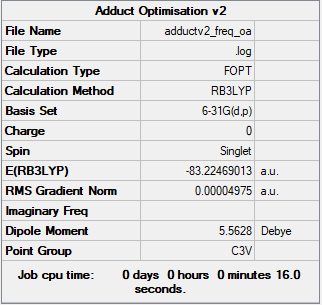
Frequency analysis log fileː Media:ADDUCTV2_FREQV2.LOG
Low frequencies --- -0.1653 -0.0665 -0.0074 11.4968 16.5035 16.5182 Low frequencies --- 263.1199 631.5063 638.9272
Optimised NH3BH3 Molecule |
Association Energy Calculation
E(NH3) = -56.55777 au
E(BH3) = -26.61532 au
E(NH3BH3) = -83.22469 au
ΔE = E(NH3BH3) - {E(NH3) + E(BH3)}
= -83.22469 - {-56.55777 + -26.61532}
= -0.0516 au
= -135 KJmol-1
The B-N dative bond formed is relatively weak when compared to the strong C-C bond in ethane (368 KJmol-1 , roughly a third of the strength [2]). However compared to hydrogen bonding (roughly 7.9 KJmol-1 [3]) the dative bond is relatively strong. For this reason I'd describe the bond as a medium strength bond.
NI3
Nitrogen triiodide
B3LYP/GEN
Full 6-31G(d,p) basis set used for N atom
LanL2DZ psuedo potential used for the 2 I atoms
NI3 Optimisation Tableː
Item Value Threshold Converged? Maximum Force 0.000066 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000476 0.001800 YES RMS Displacement 0.000324 0.001200 YES
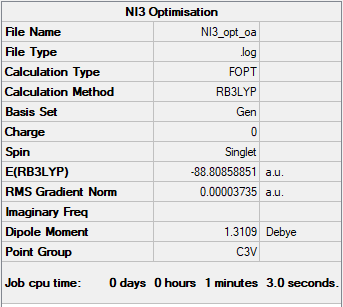
Frequency analysis log fileːMedia:NI3_FREQ_OA.LOG
Low frequencies --- -12.7332 -12.7271 -6.2836 -0.0040 0.0188 0.0634 Low frequencies --- 101.0317 101.0325 147.4105
Optimised NI3 Molecule |
Ng611 (talk) 01:48, 15 May 2019 (BST) Your NI bond length is missing!
Project Section - Ionic Liquids
[N(CH3)4]+
Tetramethylammonium ion
B3LYP/6-31G(d,p) level
[N(CH3)4] Optimisation Tableː
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000048 0.000300 YES Maximum Displacement 0.000394 0.001800 YES RMS Displacement 0.000153 0.001200 YES
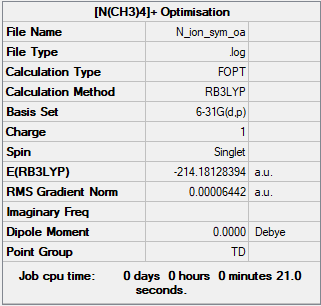
Frequency analysis log fileːMedia:N_ION_FREQ.LOG
Low frequencies --- -0.0010 -0.0009 -0.0008 18.0173 18.0173 18.0173 Low frequencies --- 184.2077 289.9327 289.9327
Optimised Tetramethylammonium Ion |
[P(CH3)4]+
Tetramethylphosphonium ion
B3LYP/6-31G(d,p) level
[P(CH3)4]+ Optimisation Tableː
Item Value Threshold Converged? Maximum Force 0.000047 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.000398 0.001800 YES RMS Displacement 0.000160 0.001200 YES
Frequency analysis log fileːMedia:P_ION_FREQ_OA.LOG
Low frequencies --- -0.0018 -0.0010 0.0008 24.2602 24.2602 24.2602 Low frequencies --- 160.2544 194.9745 194.9745
Optimised Tetramethylphosphonium Ion |
Discussion
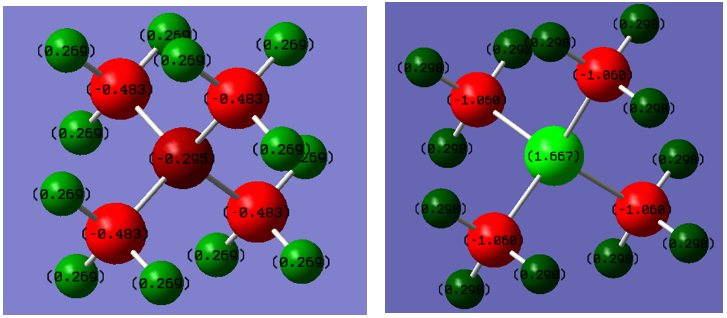
Charge Dispersion
^^ [N(CH3)4]+ ^^ ^^ [P(CH3)4]+ ^^
N = -0.295 P = 1.667
C = -0.483 C = -1.060
H = 0.269 H = 0.298
Ng611 (talk) 01:51, 15 May 2019 (BST) Numerical values look correct, but you need to make sure your colour scales are equal to one another.
The first major difference to note is the slightly negative charge distribution on the nitrogen atom (-0.295) compared to the positive charge distribution on the phosphorous atom (+1.667). This is to be expected as the smaller nitrogen atom has a higher effective charge compared to the phosphorus and pulls electron density more tightly towards it. This is reflected in their relative electronegativities on the Pauling scale, 3.04 for nitrogen and 2.15 for phosphorous[4]. Crucially the electronegativity of phosphorous is less than carbon (2.15 compared to 2.55), resulting in the carbon atoms pulling electrons away from the phosphorous. Nitrogen however is more electronegative than carbon and so the opposite occurs in [N(CH3)4]+.
Interestingly the charge distribution on hydrogen in [P(CH3)4]+ is greater than that of [N(CH3)4]+ (0.298 compared to 0.269), contradictory of the electronegativity argument. You'd expect the hydrogen bonded to the more electron deficient carbon to itself be more electron deficient. This is an example of a situation which traditional valence bond theory isn't able to explain, displaying the superiority of MO theory.
In depicting [N(R)4]+ ions the formal charge is often put on the N atom. This is since formal charge is calculated using the formula:
Formal charge = no. of valence e- - [ no. of e- in lone pairs + 1/2{no. of bonding e-}]
In the case of nitrogen in [N(CH3)4]+ (and phosphorous in [P(CH3)4]+) this results in:
Formal charge = 5 - (0 + 1/2{8}) = +1
The nitrogen is seen to have 'given up' its lone pair to form the 4th and final bond, resulting in it being given the +1 formal charge. The results of the charge analysis show that all of the positive charge is distributed amongst the hydrogen atoms and the nitrogen atom in fact has a negative charge distribution, suggesting that depicting [N(R)4]+ ions with a positive charge on the N atom is somewhat misleading.
MO Analysis
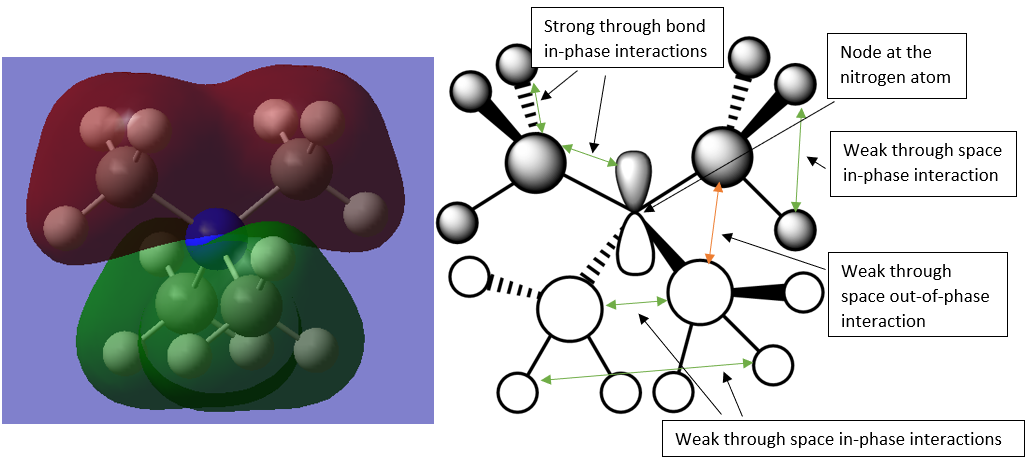
MO 7
This MO experiences a lot of strong through bond in-phase interactions as well as some weak through space interactions and the only out-of-phase interactions are through space and weak. It only contains one node which is at an atom. This orbital is overall BONDING.
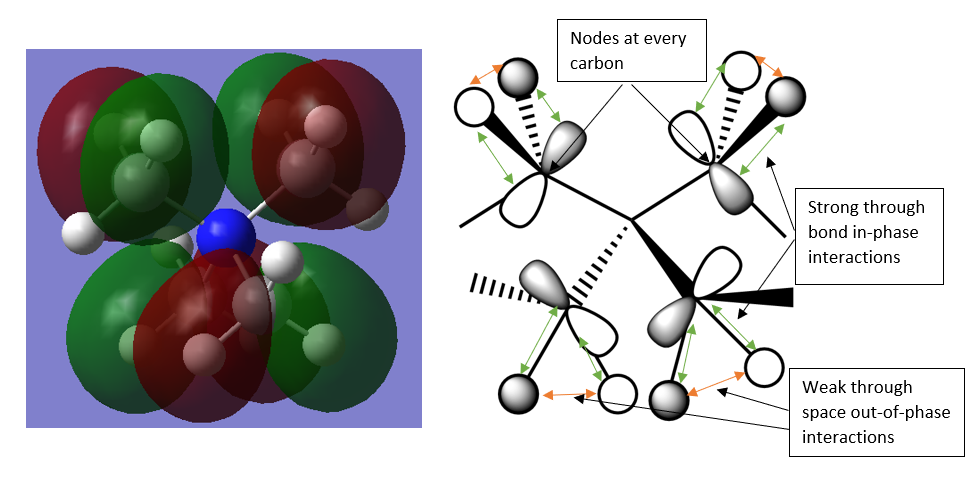
MO 16
This MO contains interactions between the carbon and hydrogen atoms of individual methyl groups but no interactions with neighbouring methyl groups nor the central nitrogen atom. This orbital is overall NON-BONDING.
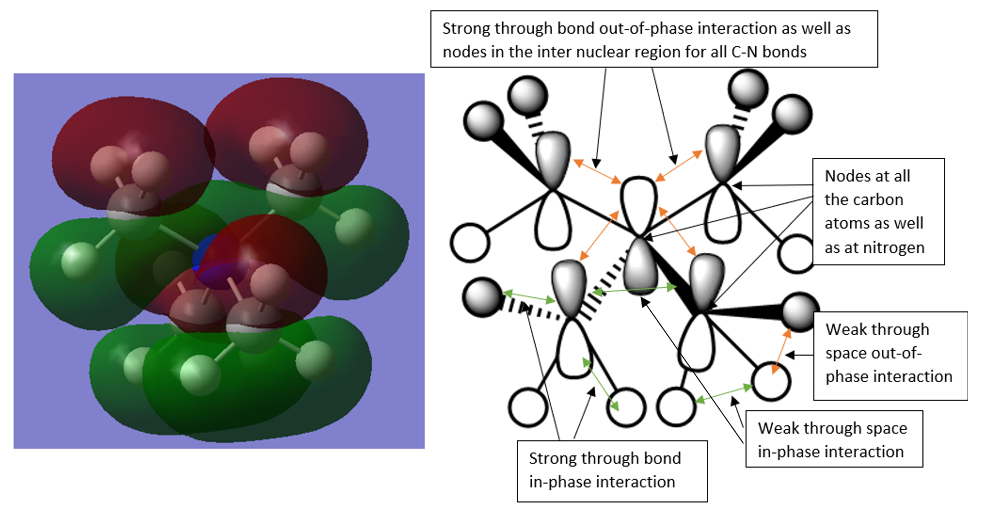
MO 22 (HOMO)
This MO contains 3 distinct nodal planes making it high energy as well as some strong through bond out-of-phase interactions. There are some in-phase interactions between the carbon and hydrogen atoms. This orbital is overall ANTI-BONDING.
Ng611 (talk) 01:55, 15 May 2019 (BST) Excellent LCAO analysis, although it would have been better form to treat your methyl group orbitals separately as frontier orbitals. Othrwise, great analysis!
References
[1] - Hunt, P. The Molecular Orbital Diagram for BH3. At http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf. Date accessed: 01/05/2019.
[2] - Stevenson, D. P. The Strengths of Chemical Bonds. J. Am. Chem. Soc. 77, 2350 (1955).
[3] - Markovitch, O. & Agmon, N. Structure and Energetics of the Hydronium Hydration Shells. J. Phys. Chem. A 111, 2253–2256 (2007).
[4] - Murphy, L. R., Meek, T. L., Allred, A. L. & Allen, L. C. Evaluation and Test of Pauling’s Electronegativity Scale. J. Phys. Chem. A 104, 5867–5871 (2000).