Rep:Mod:Newpage
Ammonia, NH3
Ammonia |
Optimisation information
NH3 optimised bond length= 1.01798 Angstroms
Literature value= 101pm [1], which is 1.01Angstroms, so the value from the optimised molecule is very accurate (the value from optimisation would be 1.02 Angstroms to 2 d.p.).
NH3 optimised bond angle=105.741°
Literature value=107.5°[2]- this means that my optimised molecule is quite accurate, as the percentage error between the 2 values is less than 1%.
Calculation type=FREQ (Optimisation)
Calculation method=RB3LYP
Point group=C3v
E(RB3LYP)= -56.55776873au
Basis set= 6-31G (d.p)
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000070 0.001800 YES RMS Displacement 0.000033 0.001200 YES RMS Displacement 0.000824 0.001200 YES
The optimisation file is linked to File:SAMUELJONES NH3 OPT COMPPHYS.LOG.
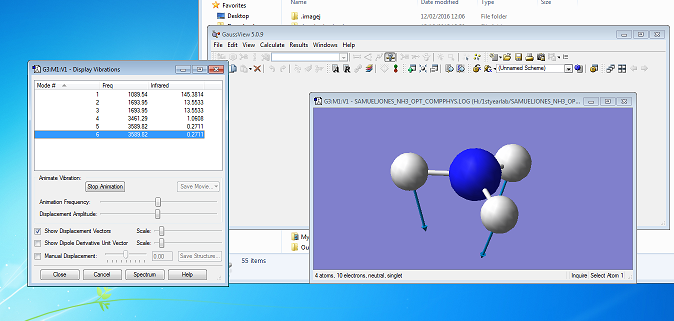
Vibrations
Vibrational modes
Using the 3N-6 rule, I would expect ammonia to have 6 modes ((3x4)-6), which it does. Modes 2 and 3 and modes 5 and 6 are degenerate, as they have the same frequency of vibration.
Vibrational mode 1=Symmetric bend
Vibrational mode 2=Asymmetric bend
Vibrational mode 3=Asymmetric bend
Vibrational mode 4=Symmetric bend
Vibrational mode 5=Asymmetric bend
Vibrational mode 6=Asymmetric bend
Mode 4 is highly symmetric, as it is a symmetric stretch of all 3 N-H bonds. The umbrella mode is probably mode 1, as the molecule looks like it turns inside-out, like an umbrella in the wind. I would expect to see 2 bands in an IR spectrum of NH3(g), as modes 2 and 3 are degenerate, but have a high enough intensity to be seen above the background and mode 1 has a very high intensity (the other modes have such low intensity that they would be lost in the background).
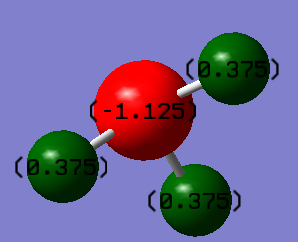
Charges and charge distribution
On the N atom, there is a charge of -1.125 and on each H, there is a charge of 0.375. I would have expected a charge of zero on both if the bond was truly covalent, but I know that N has a higher electronegativity than H, so it will draw electron density towards itself.
N2
Optimisation Information
Calculation type=FREQ (Optimisation)
Calculation method=RB3LYP
Point group=D∞h
E(RB3LYP)= -109.52412868au
Basis set= 6-31G (d.p)
Bond length= 1.10550 Angstroms
Literature value=110pm [1] ,which is 1.10 Angstroms, so the calculated bond length is very accurate.
Nitrogen |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked here File:SAMUELJONES N2 OPT 9.LOG
Vibrational mode 1=Symmetric stretch at 2457.33-1
From the 3N-5 rule, I expected there to be 1 vibrational mode (3x2)-5=1 vibrational mode (and there was).
Charge distribution
As N2 consists of 2 identical atoms with identical electronegativities, the charge on each atom is 0.
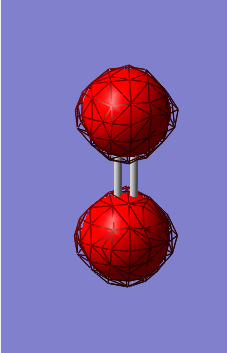
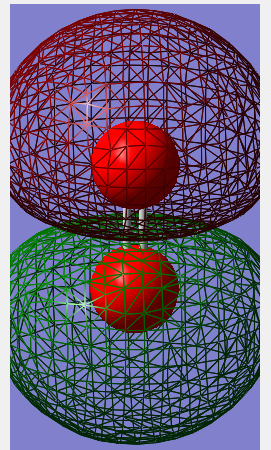
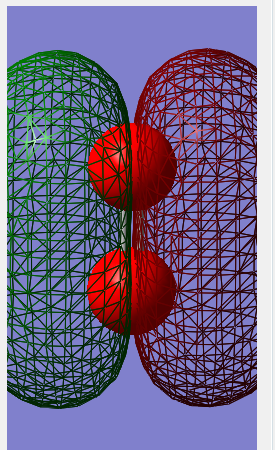
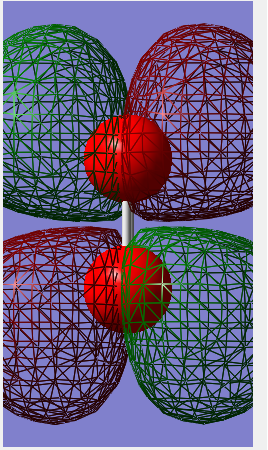
Molecular orbital diagram
 The 1σ orbital formed by mixing 2 1s orbitals. This is the smallest of many molecular orbitals that N2 has and lowest in energy.
The 1σ orbital formed by mixing 2 1s orbitals. This is the smallest of many molecular orbitals that N2 has and lowest in energy.
H2
Optimisation Information
Bond length= 0.74279 Angstroms
Literature value=74pm (0.74 Angstroms)[1], so the optimised bond length is very accurate.
Calculation type=FREQ (Optimisation)
Calculation method=RB3LYP
Point group=D∞h
E(RB3LYP)= -1.17853936au
Basis set= 6-31G (d.p)
Hydrogen |
Vibrational mode 1=Symmetric stretch of frequency 4465.68cm-1
From the 3N-5 rule, I expected there to be 1 vibrational mode (and there was).
The optimisation file is linked to Media:SAMUELJONES H2 OPT 7.LOG
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Charge distribution
As H2 consists of 2 identical atoms with identical electronegativities, the charge on each atom is 0.
The Haber-Bosch Process
N2(g)+3H2(g)--->2NH3(g)
Energy of reaction/au
E(NH3(g))=-56.55776873
2E(NH3(g))=-113.1155375
E(N2(g))=-109.52412868
E(H2(g))=-1.17853936
3E(H3(g))=-3.53561808
ΔE=2E(NH3(g))-[E(N2(g))+3E(H2(g))]=-0.05579074
Energy of reaction/kJmol-1
E(NH3(g))=-148492.4218
2E(NH3(g))=-296984.8436
E(N2(g))=-287555.5998
E(H2(g))=-3093.901528
3E(H3(g))=-9281.704584
ΔE=2E(NH3(g))-[E(N2(g))+3E(H2(g))]=-146.48 (2d.p.)
The products are more stable as the enthalpy change of the reaction is negative, which means that the bonds formed are stronger than those broken to form them.
The actual value is -91.4kJmol-1 (I found this by doubling the value given at [3](-45.7kJmol-1)). However, this was done under different conditions to those the calculations were done at. The calculations would have been done at standard temperature and pressure (298K, 1atm) but this value doesn't specify conditions, but commercially it is done with an iron catalyst at a different temperature.
Molecule of my choice
O2(g)
Information
Linear
Point group=D∞h
Charge distribution-there are no charges on the atoms, as they are both of equal electronegativity.
Optimisation
Optimised bond length=1.21602 Angstroms
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000130 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000113 0.001200 YES
Calculation type=FREQ (Optimisation)
Calculation method=RB3LYP
Point group=D∞h
E(RB3LYP)= -150.25742434au
Basis set= 6-31G (d.p)
Vibrational modes
Vibrational mode 1= 1642.74cm-1
Based on the 3N-5 rule, I expected there to be 1 vibrational modes ((3x2)-5), which there was.
Oxygen |
The optimisation file is linked to File:SAMUELJONES O2 OPT 7.LOG
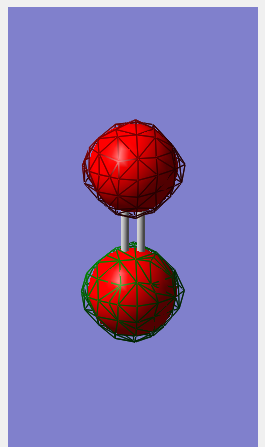
Molecular orbitals
1σ orbital
This is formed by the linear combination of 2 in phase 1s orbitals, resulting in a bonding interaction.
1σ* orbital
This is formed by the linear combination of 2 1s orbitals in anti-phase, resulting in an anti-bonding interaction.
2σ* orbital
This is formed by the linear combination of 2 2s orbitals in phase, resulting in a bonding interaction.
2σ* orbital
This is formed by the linear combination of 2 2s orbitals in anti-phase, resulting in an anti-bonding interaction.
1π orbital
This is formed by the linear combination of 2 2p orbitals in phase, resulting in a bonding interaction.
1π orbital
This is formed by the linear combination of 2 2p orbitals in phase, resulting in a bonding interaction.
1π* orbital
This is formed by the linear combination of 2 2p orbitals in anti-phase, resulting in an anti-bonding interaction.
1π* orbital
This is formed by the linear combination of 2 2p orbitals in anti-phase, resulting in an anti-bonding interaction. This is also the HOMO (highest occupied molecular orbital) and LUMO (lowest occupied molecular orbital).
The 2 π* orbitals are degenerate, so they will both be the HOMO and LUMO.
Paramagnetism
Paramagnetism is present in molecules with unpaired electrons. Oxygen has 2 unpaired electrons, so it is called a triplet (no unpaired=singlet, 1 unpaired=doublet etc.). Knowing a molecule's pairing of electrons is useful, as there are certain selection rules, which mean that only certain types of molecules can react (singlet with singlet, doublet with doublet and so on).
Paramagnetism affects the way that molecules react in an external magnetic field. This is because the unpaired electrons act as little magnets (they are spinning charges and a moving charge generates a magnetic field) and so any external magnetic field will interact with that of the unpaired electrons.
One video of this is found here https://www.youtube.com/watch?v=KcGEev8qulA#t=49. Note that N2 is also used for comparison, as it is not paramagnetic.
This is not present in singlets, as they have complete spin-pairing, which means that any magnetic field generated by 1 electron is then cancelled by the other that it is spin-paired to.
You can convert oxygen from a triplet to a singlet by adding 2 electrons to it, forming O22-, which is much more reactive, as it is extremely electronegative (it has an electronegativity of 3.44 [4] and also can now react with singlets (its reactivity is comparable to that of F2, which is also extremely electronegative (it has an electronegativity of 3.98 on the Pauling scale) [4] and a singlet).
Reactions
Group 1
With Li, the oxide forms but in excess oxygen, the compound Li2O2 can also form, as you actually form the O22- ion. This also occurs with sodium. Potassium forms the superoxide and peroxide. The other metals react to form the superoxide.[5]
Group 2
Be is unreactive due to its small size and high ionisation energy, so it doesn't form an oxide. Sr and Ba form the oxide and peroxide when reacting with O2. The other alkaline earth metals react to give the oxide.[5]
Group 13
They all react by the equation 4M(s)+3O2(g)---->2M2O3(s) Thallium also reacts at high temperatures to produce Tl2O Boron trioxide is obtained heating boric acid by 2B(OH)3----->B2O3+3H2O Thallium is the only group 13 element to form the oxide rather than the trioxide.[5]
Group 14
Their oxides are slightly acidic and their acidity decreases down the group. They all have many oxides due to their ability to expand the octet.[5]
Group 15
Nitrogen forms oxides with oxidation states ranging from -1 to +5 with all being gases at room temperature except N2O5. All of them require energy to form due to the stability of the nitrogen triple bond. Phosphorous reacts to form 2 different oxides. In limited oxygen supplies, P4O4 but in excess, it forms P4O10. On rare occasions, P4O7, P4O8 and P4O9. As, Sb and Bi all have +3 and +5 as their common oxidation states. There are other compounds like Sb4O10. Arsenic (III) oxide and antimonny(III) oxide are amphoteric whereas bismuth (III) oxide acts only as a base.[5]
Group 16
Oxygen can't expand its octet as it has no d-orbitals. It is also capable of forming double bonds due to its small size. It can react with itself to form O3 when subjected to UV light. Sulfur dioxide and trioxide are the only common sulfur oxides. Selenium and tellurium form O3, O2 and O oxides.[5]
Group 17
Fluorine adopts the -1 oxidation state, forming OF2.
Oxidation state of halogen Chlorine Bromine Iodine +1 HOCl HOBr HOI +3 HClO2 –— –— +5 HClO3 HBrO3 HIO ; HIO3 +7 HClO4 HBrO4 HIO4; H5IO6
Group 18
The noble gases are chemically inert.[5]
References
- ↑ 1.0 1.1 1.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedBond_lengths - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBond_angles - ↑ https://www.researchgate.net/publication/225769845_Haber_process_for_ammonia_synthesis accessed on 22/2/2016
- ↑ 4.0 4.1 https://www.angelo.edu/faculty/kboudrea/periodic/trends_electronegativity.htm accessed on 25/2/2016
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Main_Group_Reactions/Reactions_of_Main_Group_Elements_with_Oxygen accessed on 25/2/2016
Cite error: <ref> tag with name "Bond lengths" defined in <references> is not used in prior text.
Cite error: <ref> tag with name "Bond angles" defined in <references> is not used in prior text.