Rep:Mod:NPG11041993
Abstract
Computational chemistry has been developed over the last half-century and is now one of the most powerful methods of analyzing the dynamics of chemical reactions. The subject area is primarily occupied with obtaining numerical solutions to the Schrodinger equation, which allows for the calculation of energy for a given system of atoms and/or molecules. Calculations over an collection of different configurations for any such system will yield a potential energy hypersurface, which can be interrogated to give information about the dynamics of reactions and other processes.
In this experiment, a number of different computations are carried out to determine the energies and dynamics of three key areas in organic chemistry: the conformations of molecules; the properties of the cope rearrangement; and the stereoselectivity of a Diels-Alder reaction. Key concept in the theory of organic chemistry are applied and rationalized through the use of these computations. The results are then used to explain various phenomena associated with these three processes.
Background And Motivation
The Shrödinger Equation
The use of the Schrödinger Equation has been a key aspect of the study of atoms and molecules since its inception in 1926.
Any given system of atoms (such as a molecule, or a number of molecules) has associated with it a mathematical function, the wavefunction (Ψ), which can be interrogated to give observable information about the system. This interrogation takes place through the use of operators, and will return the product of the original wavefunction and a value for the desired observable. This is known as the eigenvalue equation:
(1)
Where is the operator, is the value of the observable, and is the wavefunction .
The Time independent Schrödinger Equation (TISE) uses one such operator - the Hamiltonian - to return the total energy of the system [1] :
(2)
Exact solutions of the Schrodinger equation for one electron systems - such as the hydrogen atom and the dihydrogen cation - are known. However, analytical solutions for multiple electron systems cannot be calculated exactly and thus must be approximated. Using successively more complicated Hamiltonian operators allows for better approximation of the energy of a molecular system. These calculations cannot be easily performed by hand and are thus must be solved iteratively using computers. Performing these calculations forms the basis of computational chemistry.[2]
The Hartree Matrix Description
Let the Hamiltonian for a multiple electron system be described as the total energy arising from three separate contributions:
- Kinetic energy
- Coulomb attraction energy
- Coulomb repulsion energies with all other electrons in the system
Thus, the Hamiltonian becomes:
(3)
where is the Hamiltonian and the terms on the right represent the operators for kinetic, attractive and repulsive energies respectively[3]. Thus the energy of the system may be extracted by operating on some Hartree orbital . This Hartree orbital can, in turn be represented using the LCAO method, becoming a linear combination of basis orbitals, thus:
(4)
Substituting equation 4 into the Schrödinger equation yields the Hartree Matrix Equation:
(5)
This equation is analogous to the eigenvalue equation outlined above. Here the energy and weighting coefficients are the eigenvalues and eigenvectors of the equation respectively. The terms and are referred to as the Hamiltonian and overlap matrices respectively.
Both the Hamiltonian matrix and must be solved. However, in order to find , one must solve the Hamiltonian matrix. Paradoxically, in order to solve the Hamiltonian matrix, one must know . This can be resolved using the self-consistent field (SCF) technique, in which an initial guess for is made. This allows for computation of the Hamiltonian matrix, which in turn allows for construction of equation 5. The equation can then be solved iteratively using the values for derived in each previous iteration. This is repeated until the difference in values between in the nth and (n+1)th iteration have become negligible. When this occurs, it is said that a self-consistent field has been derived. The final value of can be plugged back into the Hartree Matrix Equation and a value for can be extracted[4].
Additional corrections for orbital symmetry under electron interchange must be made. The combination of the Hartree Matrix Equation with symmetry corrections is referred to as the Hartree-Fock Approximation. More complex mothods (such as Density Functional Theory) exist and can be used in place of the Hartree-Fock approximation.
The Basis Orbitals (χn)
The basis set of orbitals (χn) can typically be one of three types:
- Screened Hydrogenic Orbitals
- Slater-Type Orbitals
- Gaussian-Type Orbitals
Hydrogenic orbitals are typically not used in computational chemistry and thus may be ignored entirely. Slater orbitals are typically used for linear systems of molecules and diatoms, but the integrals that result from higher order or non-linear systems cannot be computed. Gaussian type orbitals (so named, because they include a gaussian distribution function e-αr2) however are perfectly solvable for these systems and can be modified to ensure the presence of a derivative 'cusp' st their zero vector. These modified orbitals are referred to as contracted Gaussian-type orbitals and comprise the basis of the orbital sets used in the Gaussian software package[5].
Generating a Potential Energy Surface
Both the approximation method and the basis orbital set are freely selected and are collectively referred to as the level of theory at which a calculation is carried out. Larger basis sets and more intricate operators will generate more precise numerical values, but require a greater amount of processing time. Thus a balance must be struck between system resources and the desired accuracy of the answer.
Once a particular value for an equation is derived, further calculations can be conducted to find how the energy of the system would change under differing arrangements of the atoms within the system. A system with N atoms will require 3 individual co-ordinates per atom, a total of 3N co-ordinates per system. Translation and rotation of the system as a whole will not effect the energy whatsoever, and can therefore be discounted, meaning only 3N-6 co-ordinates are required. The energy can be therefore be represented as a function of these co-ordinates:
(6)
Where represents the functional form of a 'plot' resulting from the solution of the Schrodinger equation for each individual configuration of atoms . The result is a hypersurface of dimension 3N-6 which yields the energy of the system as a function of the position vectors for each atom. We define this as the potential energy hypersurface and may utilise it to analyse the dynamics of chemical reactions. [6]
Conformational study of 1,5-hexadiene
1,5-Hexadiene can exist in a number of different interchangeable conformations, with the dihedral angles between the central four carbon atoms providing the primary route of conformational interchange. The terminal carbon atoms are conformationally locked by virtue of their C=C bond and can thus be largely neglected. The resultant manifold conformers of 1,5-Hexadiene can therefore be broadly arranged into 3 types:[7]
i) Syn Conformers: The central chain of four carbon atoms are eclipsed and have a dihedral angle of approximately 0 degrees.
ii) Anti Conformers: The dihedral angle between the central carbon atoms angle is approximately 180 degrees.
iii) Gauche Conformers: Central carbon atoms have a dihedral angle of 60 degrees.
The relative energies of these three classes of conformer are governed by 3 key effects: [8]
i) σ - σ* Orbital Overlap
Overlap between the bonding σ orbital and an adjacent σ* anti bonding orbital results in an overall stabilization of the filled bonding orbital by an energy , and a destabilization of the empty anti-bonding orbital by an energy . The result is an overall stabilization of the filled natural bonding orbital (NBO, resulting in a stabilisation of the molecule itself.
ii) Pauli Repulsion
In a similar manner to the σ - σ* overlap mentioned above. If two σ bonds overlap then the resulting NBO has both bonding and anti-bonding character. The anti-bonding orbital is more destabilising than the bonding orbital is stabilising, however, and thus the result is an overall destabilization of the molecule.
Other effects - such as dispersion forces - manifest themselves in larger molecules but will largely be discounted here.
In 1,5-hexadiene, the terminal C=C bonds are fixed and therefore do not play a major role in the generation of conformers. However, the central alkyl chain is free to rotate and thus determine the overall configuration of the molecule. These four atoms can therefore be: gauche, antiperiplanar, eclipsed, or some combination of the three. We would expect a fully antiperiplanar configuration to generate the most stable species, as this would maximize σ - σ* overlap while minimizing Pauli repulsion. Conversely, the eclipsed - or synperiplanar - conformation would maximize Pauli repulsion and minimize σ - σ* overlap, resulting in maximum destabilization. The gauche conformation would lie somewhere in between the two.
Experimental
The relative stabilities of these conformers was assessed using the Gaussian software package. A 1,5-hexadiene molecule was generated with an antiperiplanar linkage between each of the 4 central carbon atoms, symmetrised and was optimised at the HF/3-21G level of theory. The resulting energy of the optimised molecule was noted. The molecule was symmetrised and re-optimised at the same level of theory, yielding a Ci point group. The process was then repeated at the same level of theory for a gauche conformer of the molecule. Vibrational calculations were then generated at the same level of theory for both molecules.
The Ci Conformer molecule was optimized again at the B3LYP/6-31G* level of theory and compared to the results of the HF/3-21G calculations.
Results and Discussion
|
|
Figure 1: The optimized conformers of 1,5,-hexadiene
| Energy (Hartrees) | |||
|---|---|---|---|
| Species | Absolute | + ZPE | Thermal Correction |
| Gauche | -231.68771615 | -231.539540 | -231.527699 |
| Anti (Ci) | -231.69253528 | -231.534385 | -231.532567 |
Figure 2: Relative energies of the HF/3-21G optimized species displayed in Figure 1
Figure 2 shows the energy of the bare potential energy surface at its minimum point, along with corrections for zero-point energy (ZPE) and thermal energy input. It can be seen that the thermally corrected energy for the anti (Ci) conformation is lower than that of the Gauche conformer. This is largely in line with the predictions outlined in the introduction, where the anti configuration maximizes σ - σ* interactions while minimizing Pauli repulsions. Furthermore the trend observed in these computations is in line with those reported by Gung et al (2010).[9] and in the lab script.
It has also reported that the global minimum for the system as a gauche conformer in which an interaction between a vinyl proton and an opposing π - orbital provides an additional stabilization energy.
| Energy (Hartrees) | |||||||
|---|---|---|---|---|---|---|---|
| HF/3-21G | B3LYP/6-31G* | ||||||
| Species | Absolute | +ZPE | Thermal Correction | Absolute | +ZPE | Thermal Correction | |
| Anti Ci | -231.699508 | -231.539540 | -231.532567 | -234.611711 | -234.469217 | -234.461867 | |
| Gauche | -231.687716 | -231.534385 | -231.527699 | -234.607883 | -234.465193 | -234.458096 | |
Figure 3: Comparison of the energies (absolute) obtained by the HF/3-21G and the B3LYP/6-31G* levels of theory
Figure 1.3 shows a comparison between the results obtained for both conformers through application of the HF/3-21G and B3LYP/6-31G* levels of theory. In every case, the results obtained by through application B3LYP/6-31G* are significantly lower (by approximately 1 Hartree) than those obtained through calculation with the HF/3-21G level of theory. This suggests that the the Hartree-Fock method significantly overestimates the energy of the molecule.
The Cope Rearrangement
The Cope Rearrangement is a widely studied pericyclic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. The reaction can occur in both directions, with the stability of both species determining the overall position of the resulting equilibrium. The mechanism for the rearrangement has been hotly debated, although the literature generally agrees that the rearrangement takes place through a concerted diradical transition state.[10] This allows for two seperate transition structures, referred to as the 'boat-like' and 'chair-like' transition structures to take place in equal proportion. Both Transition states are 6-electron [π2s + σ2s + π2s] thermally allowed processes and thus are expected to have approximately similar energies. If all else is equal, then the chair transition state is slightly favoured over that of the boat transition state.[11]
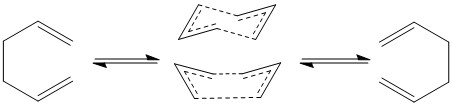
Computing a Transition State
From figure 2.1 it can be seen that the transition state consists of 2 allyl fragments arranged in the boat and chair conformation respectively. Thus it is a relatively trivial exercise to generate these fragments separately and arrange them into the appropriate conformation. An optimization to a transition state can therefore take place from this initial configuration.
The transition state may be defined as a saddle point along the minimum reaction pathway. Thus, it is a minimum with respect to the potential energy surface and a maximum with respect to the energy of the intrinsic reaction co-ordinate. The nature of the curvature of these points can thus be probed by computing the force constants at any particular reaction co-ordinate while observing that the force constants of the molecule are equal to the second derivative of the potential energy surface. Thus the nature of the force constant can be interrogated to yield information about any given reaction co-ordinate. Negative curvature of the potential energy surface (i.e a local maximum) for some given direction will generate a negative second derivative and thus will result in a negative force constant.[12]
Thus a number of different methods for computing the transition state may be utilized:
Method I:
A guess structure may be inputted and optimized to a transition state through stepwise computation of force constants. For small perturbations from the true transition structure, this method will almost always converge. However, if the guess structure is significantly different from that of the true transition structure, than no convergence will occur and the computation will fail.
Method II:
A guess structure is input, with bond making atoms set to their approximate bond making distances. These atoms are then 'frozen' (i.e locked to their present co-ordinates) and the remainder of the system is optimized. Movement of the bond making atoms is then allowed and the new structure is optimized to the transition state through calculation of force constants for all bond making atoms.
Conceptually, this optimization takes place in two phases. The first phase relaxes the molecule so the it is close to the potential energy minimum, or reaction channel. The second phase 'tracks' this potential energy minimum to the local maximum (i.e.: the transition state). This therefore has the advantage of being able to arrive at a transition state for more dissociated geometries, but may still be divergent for extremely abnormal systems.
Method III:
Both the starting molecule(s) and the product are defined. Both molecules are then optimized forwards and backwards respectively to their transition states. If the starting system and the product both converge on the same transition structure, then the transition state for this particular reaction has been arrived at. This method can be implemented through the QTS2 functionality present on Gaussian.
In this exercise, all three methods will be utilized to probe the nature of the boat and chair transition states of the cope rearrangement.
Experimental
Both transition structures were treated as 2 allyl fragments. The allyl fragment was generated and optimized to a minimum using the HF/3-21G level of theory. The optimized fragment was then copied and the two resulting fragments arranged into a boat and chair conformation. These conformations were then optimized using the three different methods outlined above:
Method I:
The molecule was optimized directly to a transition state using the Berny Algorithm at the HF/3-21G level of theory. The force constant (Hessian) matrix was computed once and the keyword Opt=NoEigen was used to prevent early termination of the calculation upon detection of imaginary negative vibrational modes.
Method II:
The terminal ends of each allyl group were frozen at approximately 2.2 Å from each other. The resulting system was then optimized at the HF/3-21G level of theory, keeping the terminal ends frozen. The bonds were unfrozen and the molecule was then optimized to a transition state at the HF/3-21G level of theory using a numerical Hessian and the Berny Algorithm.
Method III:
The precise nature of the transition state was elucidated through the use of the QTS2 method. The initial and final forms of the 1,5-hexadiene were inputted and allowed allowed to converge to a transition state.
Arrival at a transition structure was confirmed by the presence of imaginary negative frequencies which imply negative curvature of the potential energy surface.
IRC Calculations From the Transition State
Further computations were performed using the IRC functionality on Gaussian with the boat and chair transition structures utilized as a starting point. Computations were performed at the HF/3-21G level of theory. Computations were carried out in the forward direction only due to symmetric nature of the rearrangement.
Results

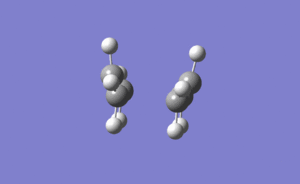
The vibrational modes of the Boat-like and chair-like transition states were confirmed through identification of imaginary negative wavelengths. Imaginary bond making vibrations at -817.96 cm-1 and -840.27 cm-1 for the chair and boat conformers respectively were identified. These are shown below.
| Energy (Hartrees) | ||||
|---|---|---|---|---|
| Method | Boat TS | Chair TS | ||
| +ZPE | Thermal Correction | +ZPE | Thermal Correction | |
| Method I | - | - | -231.466699 | -231.461340 |
| Method II | -231.450933 | -231.445305 | -231.466700 | -231.461338 |
| Method III | -234.414612 | -234.408690 | - | - |
Figure 2.2: Table showing the energies of the boat-like and chair-like transition states.
Figure 2.1 shows the energies of the the boat like and chair like transition states using the three methods outlined in the experimental. Methods I and II give very similar answers for the energy of the chair and boat transition states, with a discrepancy of approximately 0.0000001 hartrees. This strongly suggests that both methods have converged to the same transition state. Furthermore, the very low level of discrepancy suggests that both methods could be used interchangeably with little detriment to the accuracy of the final answer. A significant discrepancy exists in the energies computed using method II and method III, suggesting that one method may lack accuracy. It can also be seen that the derived transition state for the chair conformer is slightly lower in energy than that of the boat conformer.
IRC Calculations
It may be observed that the boat and chair transition states both link separate conformers of hexa-1,5-diene. Thus, one particular configuration of the starting compound will generate one specific transition structure.
Because the reaction pathway for the cope rearrangement is symmetrical, it may be observed that the one particular configuration of hexa-1,5-diene will generate one particular transition structure and vice-versa.
This is illustrated below:

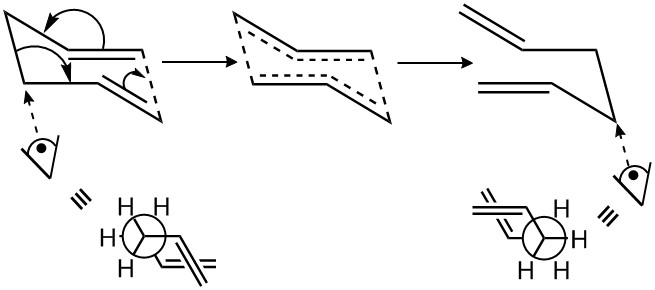
This was confirmed through the 'IRC method' outlined in the experimental. The boat and chair transition states were used as starting points and the intrinsic traction pathway was calculated in the forward direction. The symmetric nature of the cope rearrangement meant that IRC calculations could be carried out in one direction only without loss of pertinent information.
Figures 2.4 and 2.5 show the outcomes of these calculations. Viewing the animation for figure 2.4, it is easily seen that the initial result of the rearrangement is a gauche conformer, which subsequently relaxes to a lower energy state. A similar outcome is seen in Figure 2.5, in which the result of the rearrangement is the synperiplanar conformer. However, the strong Pauli repulsions and favourability of σ-σ* interactions result in a relaxation away from the synperiplanar to the gauche configuration.
The results of the IRC calculations also shed light on the favourability of the chair transition state with respect to the boat transition state. In order for the reaction to proceed via the boat transition state, the starting molecule must adopt an energetically disfavored synperiplanar conformation. This provides an additional energy requirement, thus elevating the overall energy of the transition state. A higher energy transition state results in kinetically disfavored reaction. This conforms for the calculated energies of the boat and chair transition state outlined in Figure 2.2, and with the expectations outlined in the literature. The calculated activation energies (Figure 2.5) also corroborate these observations, with the chair transition state possessing a marginally lower activation energy barrier.
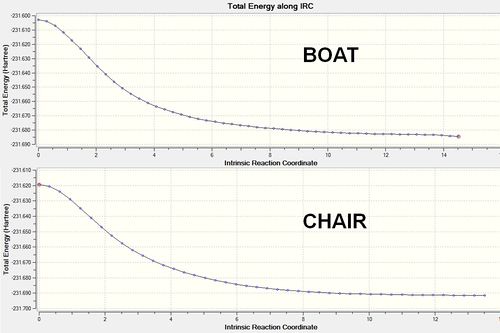
| Species | Activation Energy (Hartrees) |
|---|---|
| Boat TS | 0.08164 |
| Chair TS | 0.07225 |
Figure 2.5: Activation energies for the system derived from the IRC calculations
The Diels-Alder Reaction
Introduction and Background
The Diels-Alder reaction is one particular subset of a group of reactions known as cycloadditions. Diels-Alder reactions can be characterised by the concerted mature of the bond forming and breaking, which proceeds through a cyclic, aromatic transition structure. Thus reactions of this type are typically Hückel 4n+2 processes. Photochemical 4n process are also present but are beyond the remit of this exercise.[13]
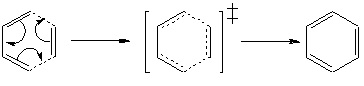
This affinity for 4n+2 electrons can be rationalized through consideration of the frontier orbitals involved in the reaction. In order for the concerted formation and breakage of bonds to occur, each interacting orbital must be of the correct parity to constructively interfere and thus form a bond. This can be further developed through consideration of the change of orbital parity over a mirror plane passing through the center of the molecule. A symmetric orbital system will not result in any parity change across the mirror plane, while an antisymmetric system will exhibit a parity change. Symmetric-Symmetric and Antisymmetric-Antisymmetric orbital interactions will always give rise to constructive interactions and thus allow the concerted formation of bonds. Symmetric-Antisymmetric interactions, however, will always possess one anti-bonding interaction and thus cannot result in concerted bond formation (stepwise bond formation, however, is still possible in certain cases).[14]
This leads to the so called Woodward-Hoffan Selection Rule, which states that:
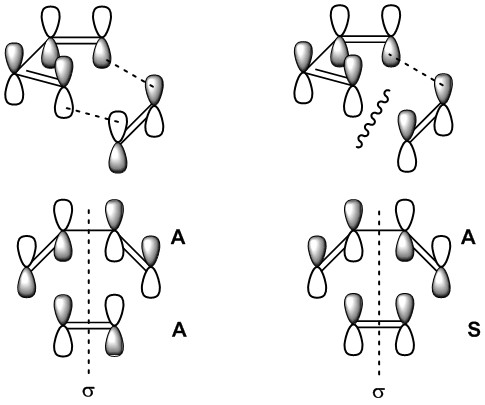
It is also vital the stereochemical outcome of the reaction. Two major modes of attack may be expected, and are referred to as the endo and exo modes of attack. These designations refer to the position of the diene relative to the attacking dienophile, as shown below.[15]
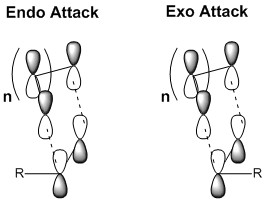
Endo and Exo attacks will result in differing stereochemistry for their respective products. The endo product will result in a trans configuration between the residual groups on the dieneophile and the central bridging carbon chain. Conversely, a product in which the central bridging carbon chain is cis to the Diels-Alder adduct.[16]
The exo product results from an exo attack, in which the orientation of reactants is less sterically hindered and is therefore expected to dominant isomer in any reaction. However, experimentally this is often not the case. The reason for this anomalous selectivity results from the constructive overlap of orbitals on the residual group on the dieneophile with the diene system. This stabilizes the transition state, thus lowering it in energy. The result in increased favourability of the endo product. This increased favourability is referred to as Alder's Endo Rule. The phenomenon of favorable orbital overlap between the diene system and dienophile resudual is dubbed the Secondary Orbital Effect [17]
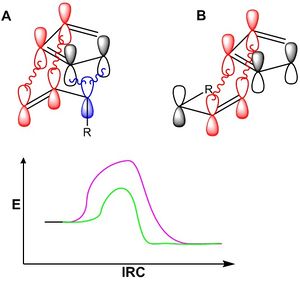
Figure 3.4 illustrates this effect. In cartoon A, the residual chain is oriented inward (i.e.: the endo position), which allows constructive secondary orbital interactions (blue) to stabilize the transition state, thus lowering the overall energy of the profile (green) compared to the violet energy profile (no secondary orbital interactions). In contrast, the exo form has no secondary orbital interactions, and thus the energy of the transitions state will be correspondingly higher. Thus, the endo product will be formed more rapidly than the exo product. Further heating however will allow interconversion between the two isomers, where the more stable exo product would be expected to dominate.
The Butadiene + Ethene Addition
Figure 3.1 shows the addition of ethene to butadiene. The product of the reaction is symmetric and thus there are no stereochemical considerations to account for in the resulting Diels-Alder Adduct. As outlined above, The HOMO and LUMO of the reactant and product must be of appropriate symmetry to permit the concerted formation of bonds. Computing of the HOMO and LUMO for butadiene and ethene will allow for prediction of the orbital symmetry and thus will allow for determination of the pericyclec nature of the reaction.
Experimental
Butadiene and Ethene were generated separately and optimized to a minimum at the B3LYP/6-31G* level of theory. The resulting HOMO and LUMO of both molecules was generated and saved as an image file. The symmetry of these orbitals was determined and plotted on an orbital correlation diagram.
The reactant system and expected product were used as inputs in a QTS2 calculation at the HF/3-21G level of theory. The computation was found to converge to a transition state and the resulting transition structure was elucidated.
Results
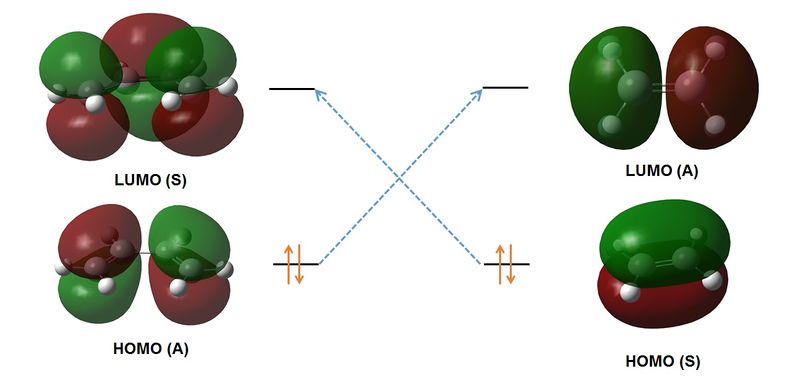
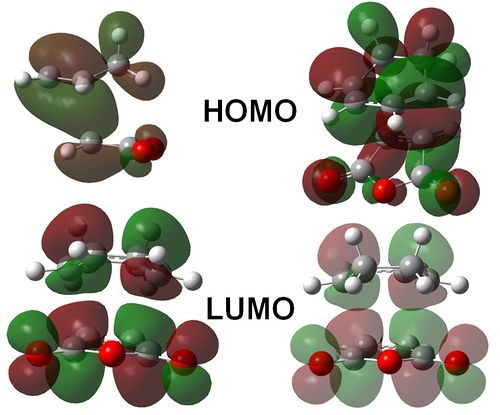
Figure 3.3 illustrates the computed HOMO and LUMO of both butadiene and ethene. It can be seen that the HOMO of each molecule is of the same symmetry as the LUMO of its counterpart. Thus the reaction is expected to be symmetry allowed. We therefore expect a cyclic, aromatic transition state for this reaction, which exhibits concerted bond formation at an imaginary frequency.
The results of this experiment are displayed below. The presence of a product forming transition state was confirmed through the presence of an imaginary bond forming frequency at -XXXX cm-1. From Figure 3.4 it is easily seen that the movement of the ethene molecule and the flanking C=C substituents on the butadiene molecule were concerted. It can therefore be inferred that a pericyclic transition state has been reached for this reaction system.
| Energy (Hartrees) | |
|---|---|
| Absolute+ZPE | +Thermal Correction |
Maleic Anhydride + Cyclopentadiene
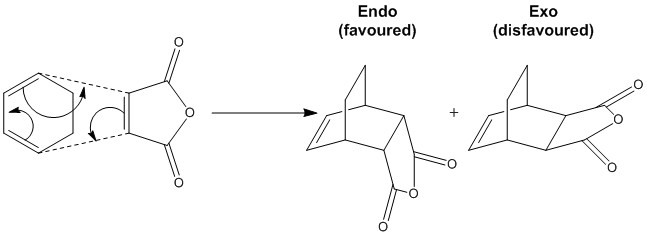
Figure 3.3 Shows the reaction between maleic anhydride and cyclohexadiene. The resulting Diels-Alder adduct has a bridging carbon chain and thus competition between the exo and endo forms will manifest. Due to secondary orbital interactions, the endo form is expected as the kinetic product, as predicted using Alder's Endo Rule. Thus, the endo transition state is likely to be lower in energy compared to the exo transition state. The energy of these transition states can then be probed using computational methods.
Method
The maleic anhydride and cyclohexadiene were generated separately and optimized at the B3LYP/6-31G** level of theory. The resulting HOMO and LUMO of both molecules was computed and saved as an image file. The symmetry of these orbitals was determined and plotted on an orbital correlation diagram.
Transition state calculations were performed using the semi-empirical AM1 Model and the Frozen Co-ordinate Method (Method II) described in Section 2. An initial guess structure for the exo and endo adducts was made. All bond making atoms were frozen through use of the 'redundant co-ordinate' functionality on Gaussian. The molecule was optimized to a minimum at the AM1 level of theory. The atoms were unfrozen and a second optimization to a transition state (Berny) was made at the same level of theory. The resulting transition structures were then optimized a second time to a TS(Berny) at the B3LYP/6-31G** level of theory, allowing free computation of force constants. The structure was found to converge and a negative frequency was found corresponding to concerted bond formation at -447.45 cm-1 and -448.45cm-1 for the endo and exo structures respectively. The orbitals of the resulting transition states were computed and saved. IRC calculations were then performed in the forward and reverse directions using the AM1 derived transition state at the AM1 level of theory.
Results
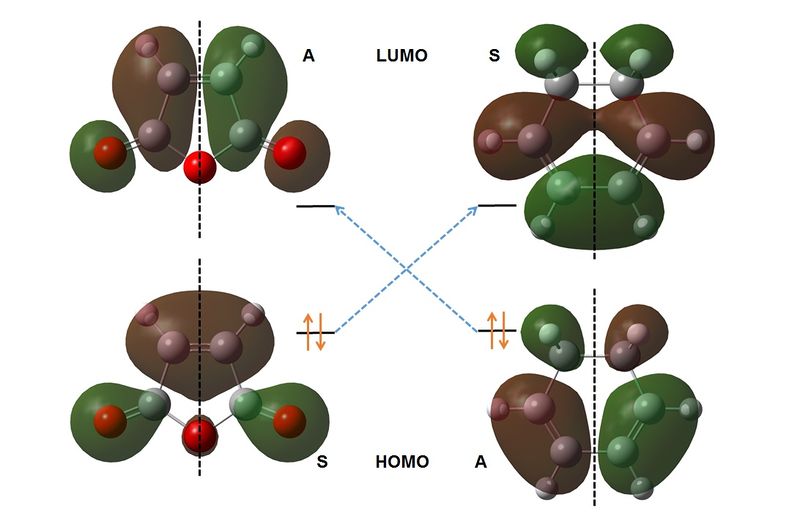

Figure 3.5 shows the Orbital Correlation diagram for the reaction outline in Figure 3.3. As was the case for the Butadiene/Ethene reaction, the HOMO of ane species and the LUMO of its counterpart are of the correct symmetry to react in a concerted fashion. Thus a pericyclic reaction is expected to take place for both the exo and endo forms. A visualization of the imaginary vibrations for the exo and endo transition states is shown in Figures 3.6 and 3.7. In each case, the observed vibration corresponds to concerted bond formation and breakage, thus confirming that the correct transition structure has been reached.
| Energy (Hartrees, Temperature Corrected) | ||
|---|---|---|
| Species | AM1 (Semi-Empirical) | B3LYP/6-31G** |
| Endo-Adduct | 0.143677 | -612.491788 |
| Exo-Adduct | 0.144881 | -612.487661 |
Figure 3.8: Tabulated thermally corrected energies of the endo and exo transition states using the AM1 and B3LYP/6-31G** levels of theory.
Figure 3.8 displays the energies of the Endo and Exo transition state. A lower energy is observed for the endo transition state, in line with Alder's Endo Rule and the predictions outlined by the secondary orbital effect. A plot of the HOMO for the endo transition state allows for visual confirmation of this effect.
Figure 3.11: Surface plots of the HOMO and LUMO of the Exo (Right) and Endo (Left) transition states. All transition state orbitals are antisymmetic with respect to a central mirror plane.
Figures 3.9 and 3.10 show visualizations of the Diels-Alder cycloaddition for the endo and exo forms of the adduct. The resulting secondary orbital interactions can be seen in figure 3.11. The inward facing anhydride group is able to interact with additional MO's on the cyclobenzadiene, thus generating a stabilizing effect. This lowers the energy of the corresponding transition state, thus accounting for the discrepancy in energy of the two forms.
Figure 3.12 displays the lengths of the bonds affected as the reaction system approaches the transition structure. The C-C bonds between maleic anhydride and the cyclohexadiene molecule are much longer than typical C-C bonds, which have a length of approximately 154pm. This large discrepancy is most likely an artifact of the 'Frozen Co-ordinate method' and should not be taken as indicative of the true mechanism to the reaction. An elongation of double bonds is observed, with double bond distances averaging around 140nm, part way in between those of a C-C single bond and a C-C double bond, suggesting the conversion of a double bond to a single bond. The bond lengths are similar in size to those of aromatic C-C bonds, suggesting the possibility of an aromatic transition state.
|
|
Figure 3.11: JMOl files of the endo and exo isomer displaying the distances of the bonds that are being made and broken.
Conclusion
In the first exercise, the relative stabilities of a gauche and an antiperiplanar conformation of hexa-1,5-diene were studied. The gauche and antiperiplanar conformers were found to have C2 and Ci symmetry respectively, and the gauche C2 conformer was found to be significantly higher in energy compared to the Anti Ci conformer, suggesting that the gauche conformation is significantly less stable than its counterpart. Furthermore, it was shown that the results obtained by the HF/3-21G level of theory significantly overestimate the energy of the optimized structure. This in turn suggests that more powerful methods and larger basis sets will be needed to generate more numerically precise answers.
In the second excercise, three idfferent methods were utilised to probe the nature of a transition structure. These methods were found to yield vary similar results and could therefore be used interchangeably. The activation energy for the cope rearrangement was computed and the results were found to conform to theory.
In the third exercise, two Diels-Alder reactions were studied. The transitions states for both reactions were found and confirmed. Additional calculations demonstrated the favourability of the endo transition state over the exo transition state, in keeping with Alder's rule. The Molecular Orbitals were also calculated and found to agree with the Woodward-Hoffman selection rules, in addition to permitting rationalization of the secondary orbital effect.
These exercises have demonstrated the power of computational chemistry in providing information about molecules and chemical reactions, enabling one to retrieve data about a molecule that could not be easily determined experimentally without leaving one's desk.
Citations
- ↑ Hinchliffe, Alan. Computational Quantum Chemistry. p.3, Chichester: Wiley, 1988. Print.
- ↑ Hinchliffe, Alan. Computational Quantum Chemistry. p.12, Chichester: Wiley, 1988. Print.
- ↑ Hinchliffe, Alan. Computational Quantum Chemistry. p.19, Chichester: Wiley, 1988. Print.
- ↑ Hinchliffe, Alan. Computational Quantum Chemistry. p.21, Chichester: Wiley, 1988. Print.
- ↑ Hinchliffe, Alan. Computational Quantum Chemistry. p.22, Chichester: Wiley, 1988. Print.
- ↑ Leach, Andrew R. Molecular Modelling: Principles and Applications. p.4, Harlow, England: Prentice Hall, 2001. Print.
- ↑ Isaacs, Neil S. Physical Organic Chemistry. p.341 Harlow: Longman, 1995. Print.
- ↑ Isaacs, Neil S. Physical Organic Chemistry. p.342 Harlow: Longman, 1995. Print.
- ↑ doi:10.1021/ja00111a016
- ↑ Clayden, Jonathan. Organic Chemistry. Oxford: Oxford UP, 2001. Print.
- ↑ Fleming, Ian. Pericyclic Reactions. p. 78 Oxford: Oxford UP, 1999. Print.
- ↑ Leach, Andrew R. Molecular Modelling: Principles and Applications. p.5, Harlow, England: Prentice Hall, 2001. Print.
- ↑ Fleming, Ian. Pericyclic Reactions. p.32 Oxford: Oxford UP, 1999. Print.
- ↑ Fleming, Ian. Pericyclic Reactions. p.33 Oxford: Oxford UP, 1999. Print.
- ↑ Fleming, Ian. Pericyclic Reactions. p.8 Oxford: Oxford UP, 1999. Print.
- ↑ Fleming, Ian. Pericyclic Reactions. p.8 Oxford: Oxford UP, 1999. Print.
- ↑ doi:jo00384a016