Rep:Mod:NF333mp
Mini Project: Ionic liquids
Comparison of selected 'onium' cations
Optimisation of [N(CH3)4]+

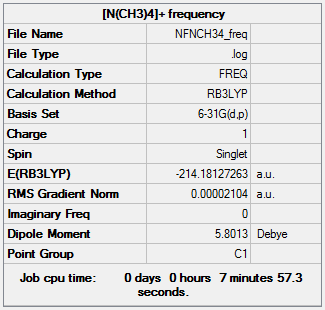
The optimisation file can be found here and this is the
Dspace-URL.
Item Value Threshold Converged? Maximum Force 0.000074 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.001369 0.001800 YES RMS Displacement 0.000363 0.001200 YES Predicted change in Energy=-5.564733D-08 Optimization completed. -- Stationary point found.
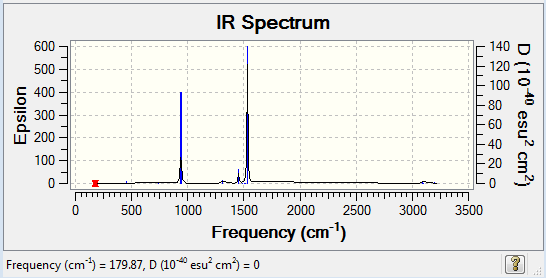
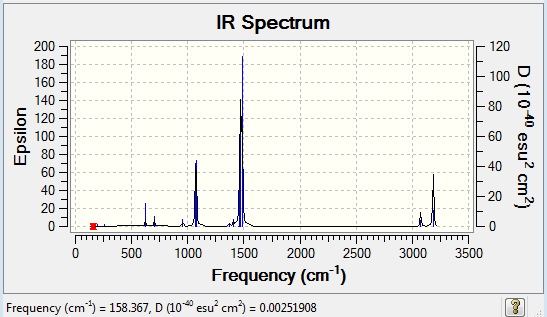
Frequency analysis of [N(CH3)4]+
The frequency file can be found here and this is the Dspace-URL.
Low frequencies --- -13.0244 -0.0008 -0.0004 0.0007 6.1739 11.9602 Low frequencies --- 179.8943 278.8652 285.7089
| Number | frequency | intensity | Number | frequency | intensity | Number | frequency | intensity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 179 | 0 | 16 | 1076 | 0 | 31 | 1530 | 54 | ||
| 2 | 279 | 0 | 17 | 1182 | 0 | 32 | 1531 | 54 | ||
| 3 | 286 | 0 | 18 | 1183 | 0 | 33 | 1533 | 53 | ||
| 4 | 293 | 0 | 19 | 1306 | 1 | 34 | 3087 | 1 | ||
| 5 | 358 | 0 | 20 | 1306 | 1 | 35 | 3087 | 1 | ||
| 6 | 360 | 0 | 21 | 1307 | 1 | 36 | 3088 | 1 | ||
| 7 | 453 | 0 | 22 | 1452 | 5 | 37 | 3096 | 0 | ||
| 8 | 454 | 0 | 23 | 1454 | 5 | 38 | 3188 | 0 | ||
| 9 | 455 | 0 | 24 | 1454 | 5 | 39 | 3188 | 0 | ||
| 10 | 736 | 0 | 25 | 1486 | 0 | 40 | 3188 | 0 | ||
| 11 | 939 | 22 | 26 | 1486 | 0 | 41 | 3189 | 0 | ||
| 12 | 939 | 22 | 27 | 1501 | 0 | 42 | 3190 | 0 | ||
| 13 | 940 | 22 | 28 | 1502 | 0 | 43 | 3194 | 1 | ||
| 14 | 1075 | 0 | 29 | 1511 | 0 | 44 | 3195 | 1 | ||
| 15 | 1075 | 0 | 30 | 1530 | 0 | 45 | 3195 | 1 |
All the vibrational frequencies are positive and thus a minimum has been obtained.
NBO analysis of [N(CH3)4]+
The NBO analysis file can be found here and this is the Dspace-URL.
Optimisation of [P(CH3)4]+
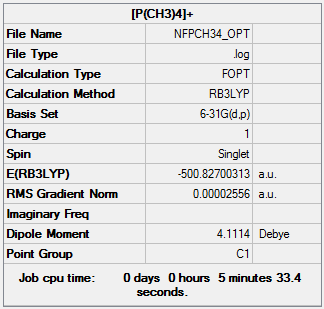
The optimisation file can be found here and this is the
Dspace-URL.
Item Value Threshold Converged?
Maximum Force 0.000148 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000899 0.001800 YES
RMS Displacement 0.000305 0.001200 YES
Predicted change in Energy=-1.785060D-07
Optimization completed.
-- Stationary point found.
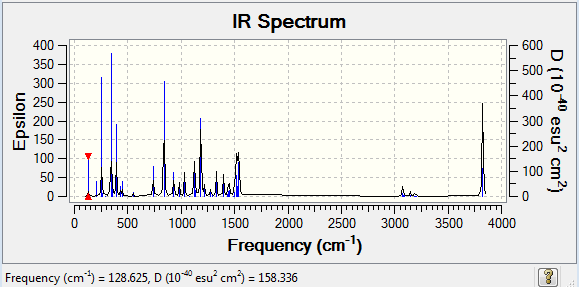
Frequency analysis of [P(CH3)4]+
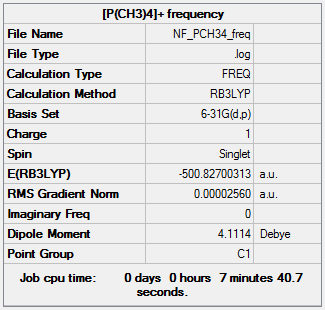
The frequency file can be found here and this is the
Dspace-URL.
Low frequencies --- -16.5110 -0.0012 0.0019 0.0028 4.9692 16.2247 Low frequencies --- 153.3828 183.0512 191.0039
NBO analysis of [P(CH3)4]+
The NBO analysis file can be found here and this is the Dspace-URL.
The positive charge on phosphorus can be explained by several factors. It donates its lone pair to form one of the P-C bonds and thus decreases its negative charge. So it is electron deficient. In contrast to [N(CH3)4]+ however, phosphorus has more diffuse orbitals than nitrogen does so that the orbital overlap between carbon and phosphorus is worse. In addition phosphorus is also less electronegative than carbon. Therefore phosphorus is not able to effectively compensate its electron deficiency. It stays positively charges.
BD ( 1) C 1 - P 17 ( 59.56%) 0.7718* C 1 s( 25.24%)p 2.96( 74.68%)d 0.00( 0.08%) ( 40.44%) 0.6359* P 17 s( 24.99%)p 2.97( 74.16%)d 0.03( 0.85%) To balance some of the overall positive charge of this molecule all carbon atoms are negatively charged. They do this by withdrawing electron density from the surrounding hydrogen atoms. BD ( 1) C 1 - H 2 ( 64.78%) 0.8049* C 1 s( 24.88%)p 3.02( 75.07%)d 0.00( 0.04%) ( 35.22%) 0.5934* H 2 s( 99.95%)p 0.00( 0.05%) The hydrogen atoms are positively charged because they do not have much electron density in the first place. In addition, due to the good C-H orbital overlap the slightly more electronegative carbon pulls off electron density. This is why carbon is actually the most negatively charged.
|
Optimisation of [S(CH3)3]+
The optimisation file can be found here and this is the
Dspace-URL.
Item Value Threshold Converged?
Maximum Force 0.000040 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.000519 0.001800 YES
RMS Displacement 0.000142 0.001200 YES
Predicted change in Energy=-1.109390D-08
Optimization completed.
-- Stationary point found.
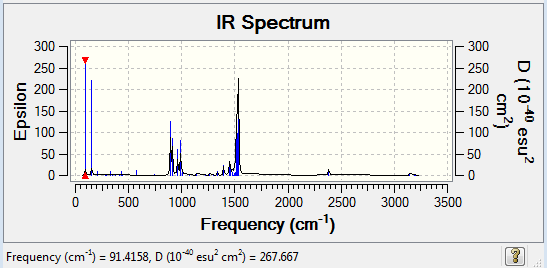
Frequency analysis of [S(CH3)3]+
The frequency file can be found here and this is the Dspace-URL.
Low frequencies --- -12.9857 -8.1326 -0.0022 0.0025 0.0030 22.9251 Low frequencies --- 158.4653 194.1206 198.5766
NBO analysis of [S(CH3)3]+
The NBO analysis file can be found here and this is the Dspace-URL.
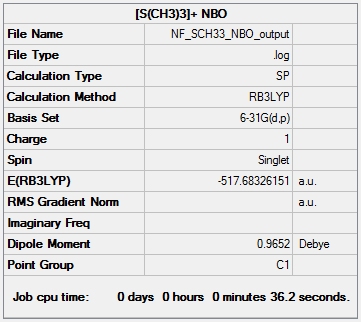
The positive charge on sulfur can be explained by several factors. It donates one of its lone pair into the S-C bonds and thus decreases its negative charge. In contrast to [N(CH3)4]+ however, sulfur has more diffuse orbitals than nitrogen so that the orbital overlap between carbon and sulfur is worse. In addition sulfur is almost as electronegative as carbon. Therefore sulfur is not able to effectively compensate its electron deficiency. It stays positively charged.
BD ( 1) C 1 - S 13 ( 48.67%) 0.6976* C 1 s( 19.70%)p 4.07( 80.16%)d 0.01( 0.14%) ( 51.33%) 0.7165* S 13 s( 16.95%)p 4.86( 82.42%)d 0.04( 0.63%) To balance some of the overall positive charge of this molecule all carbon atoms are negatively charged. They do this by withdrawing electron density from the surrounding hydrogen atoms. BD ( 1) C 1 - H 2 ( 64.82%) 0.8051* C 1 s( 26.50%)p 2.77( 73.45%)d 0.00( 0.05%) ( 35.18%) 0.5931* H 2 s( 99.95%)p 0.00( 0.05%) The hydrogen atoms are positively charged because they do not have much electron density in the first place. In addition, due to the good C-H orbital overlap the slightly more electronegative carbon pulls off electron density.
|
Comparison of [N(CH3)4]+,[P(CH3)4]+ and [S(CH3)3]+
| Element | N | S | C | P | H |
|---|---|---|---|---|---|
| Electronegativity | 3.0 | 2.5 | 2.5 | 2.1 | 2.2 |
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | Interpretation | |
|---|---|---|---|---|
| C-X | 1.51 (lit. 1.451[2]) | 1.81 (lit. 1.841[3]) | 1.82 (lit. 1.83[4]) | The bond length order of N-C < P-C < S-C can be rationalised by the fact that nitrogen is the smallest and most electronegative atom. Sulfur and phosphorus are both in the second period and their bond to C is of almost equal length. Even though sulfur has a greater electronegativity S-C is slightly longer. This can be reasoned by the electron-electron repulsion from sulphur's lone pair or considering the orbital overlap. Nitrogen and carbon are in the same period and so their orbitals overlap very well while those of phosphorus and sulfur are more diffuse. The worse the overlap the longer is the bond. All computed bond lengths are close to the literature value. The angles are except for minor differences very similar. Just the C-S-C angle is quite different from the other two, which is due to its lone pair. Sulphur is in group 6 rather than 5 like the others and thus three methyl groups are already sufficient to form the cation. One lone pair is left for the S atom while the one in nitrogen and phophorus is used for the fourth methyl group. A lone pair of electrons blocks more space than electrons bonding to a methyl group, squeezing the three methyls on sulphur a bit more. Ergo the angle is smaller. |
| C-H | 1.09 | 1.09 | 1.09 | |
| C-X-C | 109.5° | 109.5° | 102.7° | |
| H-C-X | 108.9° | 109.9° | 110.6° | |
| H-C-H | 110.1° | 109.0° | 109.4° |
| Molecule | Orbital contribution | Interpretation |
|---|---|---|
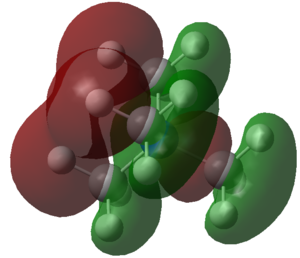
| [N(CH3)4]+ | BD ( 1) C 1 - N 17 ( 33.65%) 0.5801* C 1 s( 20.76%)p 3.81( 79.07%)d 0.01( 0.16%) ( 66.35%) 0.8146* N 17 s( 25.00%)p 3.00( 74.97%)d 0.00( 0.03%) |
The data obtained gives information about the orbital contributions of both atoms to their bond. Following on from the interpretation above it can be noticed that sulfur has a higher p-orbital contribution to the a S-C bond than nitrogen or phosphorus do. Since all three p-orbitals are aligned 90° to each other this also accounts for the fact that the C-S-C angles in [S(CH3)3]+ are smaller — closer to 90° — than those of the other two compounds. While nitrogen contributes about two third to the C-N bond, S-C is of almost equal contribution with sulphur's being slightly higher than carbons. Phosphorus, however, contributes quite some electron density less to the P-C bond than does carbon. This can be mainly explained by the electronegativity order: N > S > C > P . Nitrogen is much more electronegative than carbon and thus the electron density of the N-C bond is situated closer to N than to C. This inductive effect is almost balanced in the S-C bond and shifts towards carbon when swapping from S to P. |
| [P(CH3)4]+ | BD ( 1) C 1 - P 17 ( 59.56%) 0.7718* C 1 s( 25.24%)p 2.96( 74.68%)d 0.00( 0.08%) ( 40.44%) 0.6359* P 17 s( 24.99%)p 2.97( 74.16%)d 0.03( 0.85%) | |
| [S(CH3)3]+ | BD ( 1) C 1 - S 13 ( 48.67%) 0.6976* C 1 s( 19.70%)p 4.07( 80.16%)d 0.01( 0.14%) ( 51.33%) 0.7165* S 13 s( 16.95%)p 4.86( 82.42%)d 0.04( 0.63%) |
Influence of functional groups
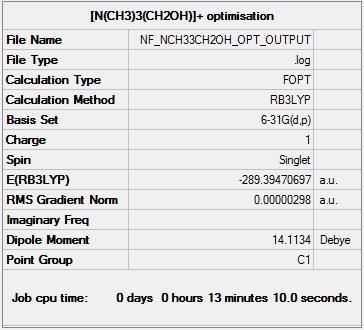
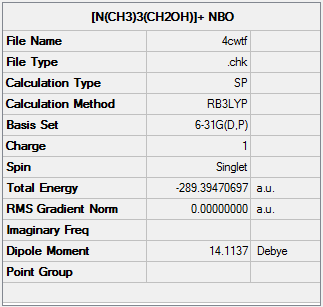
Optimisation of [N(CH3)3(CH2OH)]+
The optimisation file can be found here.
Item Value Threshold Converged? Maximum Force 0.000004 0.000015 YES RMS Force 0.000001 0.000010 YES Maximum Displacement 0.000036 0.000060 YES RMS Displacement 0.000009 0.000040 YES Predicted change in Energy=-1.020218D-10 Optimization completed. -- Stationary point found.
Frequency analysis of [N(CH3)3(CH2OH)]+
The frequency file can be found here.
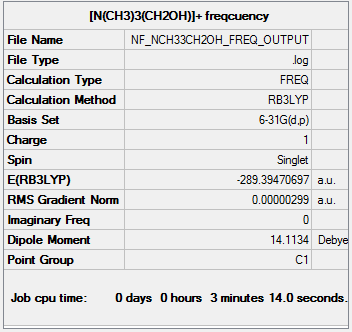
Low frequencies --- -14.4774 -5.6007 0.0010 0.0011 0.0014 10.3045 Low frequencies --- 128.6428 210.7614 255.3099
NBO analysis of [N(CH3)3(CH2OH)]+
The NBO analysis file can be found here.
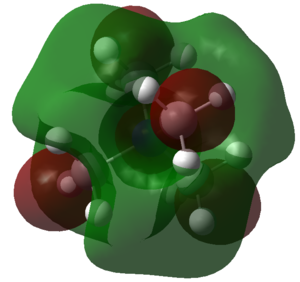
| Charge Distribution of [N(CH3)3(CH2OH)]+ |
|---|
 |
| Bright red colour indicates negative charge, bright green colour positive charge. Charge range: -0.500 to 0.500
|
| N atom | O atom | C atoms | H atoms | ||||
|---|---|---|---|---|---|---|---|
| 16 | -0.322 | 17 | -0.725 | 1 | -0.492 | 2 | 0.265 |
| 5 | -0.494 | 3 | 0.274 | ||||
| 9 | 0.089 | 4 | 0.269 | ||||
| 12 | -0.491 | 6 | 0.271 | ||||
| 7 | 0.262 | ||||||
| 8 | 0.272 | ||||||
| 10 | 0.249 | ||||||
| 11 | 0.237 | ||||||
| 13 | 0.265 | ||||||
| 14 | 0.266 | ||||||
| 15 | 0.282 | ||||||
| Here the charges are not all the the same for every atom of the same element. This is due to the reduced symmetry compared to the first three examples. The negative charge on nitrogen is obviously due to its great electronegativity, which is nicely shown by the contribution nitrogen does to the N-C(5) bond i.e. the percentage of the electron density of the bond that is situated on nitrogen. This will be almost the same figures for C(1) and C(12). BD ( 1) C 5 - N 16 ( 34.06%) 0.5836* C 5 s( 20.81%)p 3.80( 79.03%)d 0.01( 0.16%) ( 65.94%) 0.8120* N 16 s( 25.47%)p 2.93( 74.50%)d 0.00( 0.03%) However, the N atom donates its lone pair to form one of the N-C bonds and thus decreasing its negative charge. Nitrogen's contribution is slightly higher in its bond to C(9) because carbon 9 already looses so much electron density to the neighboring oxygen thus it is easier for nitrogen to withdraw more electron density relative to the amount it sucks off the other bonded carbons. BD ( 1) C 9 - N 16 ( 32.72%) 0.5720* C 9 s( 20.27%)p 3.92( 79.55%)d 0.01( 0.18%) ( 67.28%) 0.8203* N 16 s( 23.47%)p 3.26( 76.50%)d 0.00( 0.03%) The oxygen atom is the most negatively charged since it has the greatest electronegative of all elements in this molecule and can suck off electron density from its neighboring atoms very effectively due to a good orbital overlap. Both carbon and hydrogen are in the same period as oxygen. Therefore O has a greater bond contribution to the O-C bond than carbon. BD ( 1) C 9 - O 17 ( 33.90%) 0.5822* C 9 s( 23.72%)p 3.21( 76.04%)d 0.01( 0.24%) ( 66.10%) 0.8130* O 17 s( 32.28%)p 2.10( 67.64%)d 0.00( 0.08%) Consequently, the electron suction from both oxygen and nitrogen results in carbon atom 9 to have the most positive charge relative to the other C atoms. LP ( 2) O 17 /144. BD*( 1) C 9 - N 16 18.99 0.51 0.088 Interesting is also the fact that oxygen donates one of its lone pair into the C(9)-N(16) antibonding orbital weakening this bond and providing partial delocalisation. |
Optimisation of [N(CH3)3(CH2CN)]+

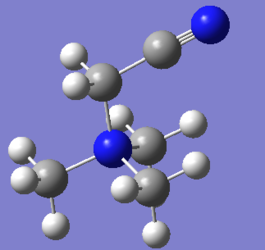
The optimisation file can be found here and this is the
Dspace-URL.
Item Value Threshold Converged?
Maximum Force 0.000023 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000661 0.001800 YES
RMS Displacement 0.000148 0.001200 YES
Predicted change in Energy=-7.074140D-09
Optimization completed.
-- Stationary point found.
Frequency analysis of [N(CH3)3(CH2CN)]+
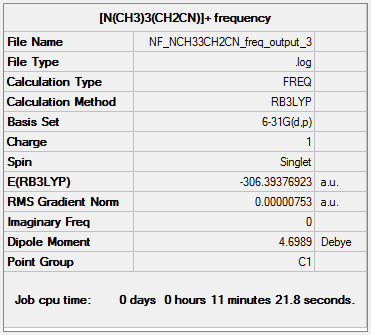
The frequency file can be found here and this is the
Dspace-URL.
Low frequencies --- -12.0745 -0.0009 -0.0006 0.0002 5.4144 13.3080 Low frequencies --- 91.4571 153.9675 210.0185
NBO analysis of [N(CH3)3(CH2CN)]+
The NBO analysis file can be found here and this is the Dspace-URL.
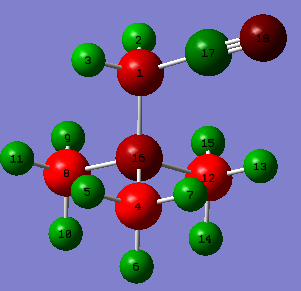
| Charge Distribution of [N(CH3)3(CH2CN)]+ |
|---|
 |
| Bright red colour indicates negative charge, bright green colour positive charge. Charge range: -0.500 to 0.500
|
| N atoms | C atoms | H atoms | |||
|---|---|---|---|---|---|
| 16 | -0.289 | 1 | -0.358 | 2 | 0.309 |
| 18 | -0.186 | 4 | -0.489 | 3 | 0.309 |
| 8 | -0.485 | 5 | 0.270 | ||
| 12 | -0.489 | 6 | 0.274 | ||
| 17 | 0.209 | 7 | 0.282 | ||
| 9 | 0.271 | ||||
| 10 | 0.277 | ||||
| 11 | 0.271 | ||||
| 13 | 0.282 | ||||
| 14 | 0.282 | ||||
| 15 | 0.269 | ||||
| Here the charges are not all the the same for every atom of the same element. This is due to the reduced symmetry compared to the first three examples. The negative charge on nitrogen 16 is obviously due to its great electronegative. Ergo more electron density of for example the N(16)-C(4) bond is on nitrogen. These figures will be very similar in the C(8)-N(16) and C(12)-N(16) bonds. BD ( 1) C 4 - N 16 ( 33.12%) 0.5755* C 4 s( 20.23%)p 3.93( 79.60%)d 0.01( 0.17%) ( 66.88%) 0.8178* N 16 s( 25.36%)p 2.94( 74.61%)d 0.00( 0.03%) However, it donates its lone pair to form one of the N-C bonds and thus decreasing its negative charge. It becomes electron deficient. Nitrogen atom 18 is negatively charged since it withdraws electron density from carbon 17. BD ( 1) C 17 - N 18 ( 42.68%) 0.6533* C 17 s( 47.95%)p 1.09( 52.03%)d 0.00( 0.02%) ( 57.32%) 0.7571* N 18 s( 45.15%)p 1.21( 54.49%)d 0.01( 0.36%) It can be noticed that the contribution of N(18) to the N(18)-C(17) bond is less than that of N(16)-C(4), which is due N(18) lacking electron deficiency. Also nitrogen atom 18 can suck off electron density only from its neighboring carbon atom 17 while N(16) has 4 surrounding carbon atoms. Therefore nitrogen atom 16 is more negatively charged than 18. BD ( 1) C 17 - N 18 ( 42.68%) 0.6533* C 17 s( 47.95%)p 1.09( 52.03%)d 0.00( 0.02%) ( 57.32%) 0.7571* N 18 s( 45.15%)p 1.21( 54.49%)d 0.01( 0.36%) BD ( 2) C 17 - N 18 ( 47.13%) 0.6865* C 17 s( 0.00%)p 1.00( 99.95%)d 0.00( 0.05%) ( 52.87%) 0.7271* N 18 s( 0.00%)p 1.00( 99.59%)d 0.00( 0.41%) BD ( 3) C 17 - N 18 ( 49.36%) 0.7026* C 17 s( 0.09%)p99.99( 99.87%)d 0.45( 0.04%) ( 50.64%) 0.7116* N 18 s( 0.03%)p99.99( 99.57%)d15.47( 0.40%) Furthermore,
The hydrogen atoms are positively charged because they do not have much electron density in the first place. In addition, due to the good C-H orbital overlap the slightly more electronegative carbon pulls off their electron density. This is why carbon is actually the most negatively charged.
27. LP ( 1) N 18 /122. RY*( 1) C 17 16.69 1.20 0.127 27. LP ( 1) N 18 /145. BD*( 1) C 1 - C 17 12.71 0.94 0.098 |
Comparison of [N(CH3)4]+,[N(CH3(CH2OH)3]+ and [N(CH3)3(CH2CN)]+
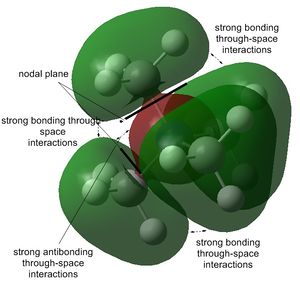
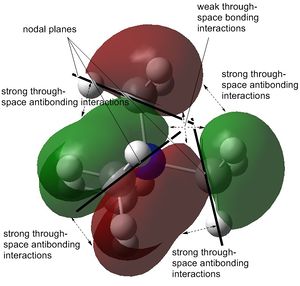
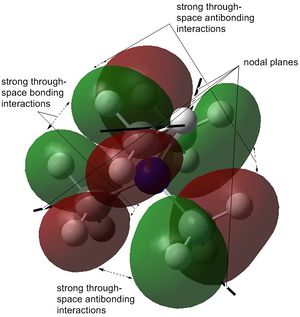
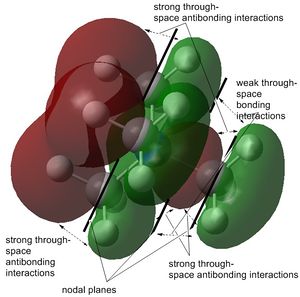
The stability order of the HOMOs (starting with the most stable) is the following: [N(CH3)4]+ > [N(CH3)3(CH2CN)]+ > [N(CH3)3(CH2OH)]+.
This is because the one from [N(CH3)4]+ is weakly bonding as already shown above. It has π-bonding interactions and nodes are only at atoms through p-orbitals. [N(CH3)3(CH2CN)]+ is almost non-bonding except for two π-antibonding interactions of which the biggest would be the nitrile group interacting with the CH2 carbon out-of-phase. However, the nitrile functional group itself has a large electron density interacting in-phase, probably making the overall molecule very slightly bonding. The mostly antibonding interactions in [N(CH3)3(CH2OH)]+ continue throughout the molecule. The only in-phase overlap worth mentioning is that of oxygen's lone pair with the H s-orbitals and C p-orbital of CH2.
The stability order of the LUMOs (starting with the most stable) is the following: [N(CH3)3(CH2CN)]+ > [N(CH3)4]+ > [N(CH3)3(CH2OH)]+.
This is because the one of [N(CH3)3(CH2CN)]+ has a nice interaction of the nitrile carbon with the CH2 carbon in a π-bonding manner and overlaps also with p-orbitals of all other carbons. The large difference to the HOMO, however, is obvious since many other antibonding interactions such as the out-of-phase central nitrogen are present. [N(CH3)4]+ and [N(CH3)3(CH2OH)]+ are lower in energy than [N(CH3)3(CH2CN)]+ because of the to the strong through-space antibonding interactions and the internuclear node between the central nitrogen atom and the surrounding methyl carbons.[N(CH3)3(CH2OH)]+ however, is the most destabilised one since it has stronger antibonding interactions, which are due to the in-phase overlap of the oxygen, CH2 and nitrogen orbitals with all other orbitals around.
The nitrile functional group makes the acceptance of electron density more profitable while an alcohol functional group results in more destabilisation relative to [N(CH3)4]+. Conversely, the alcohol group makes donation of electron density easier. However, this is rather hypothetical considering that these compounds are cations already.
The HOMO-LUMO gap gives information about the conjugation of each ion. The smaller the gap, the greater the degree of conjugation. Also the smaller the gap the easier electrons can be promoted to excited states.
- ↑ Atkin's Physical Chemistry, 4th edition
- ↑ J.E. Wollrab and V.W. Laurie, J. Chem. Phys., 1969, 51, 1583 DOI:10.1063/1.1672214
- ↑ D.R. Lide and D.E. Mann, J. Chem. Phys., 2004, 29, 914 DOI:10.1063/1.1744611
- ↑ D.E. Zuccaro and J.D. McCullough, Zeitschirft für Kristallog., 2010, 112, 401 DOI:10.1524/zkri.1959.112.1-6.401