Rep:Mod:NBC1234
Week One
Day One
3-21G
The BH3 optimisation file is linked to here
| Q | A |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -26.46226338 ±0.003808780 a.u. |
| Gradient | 0.00020672 a.u. |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 14.0 seconds |
The corresponding log book:
Item Value Threshold Converged? Maximum Force 0.000413 0.000450 YES RMS Force 0.000271 0.000300 YES Maximum Displacement 0.001610 0.001800 YES RMS Displacement 0.001054 0.001200 YES Predicted change in Energy=-1.071764D-06 Optimization completed.
6-31G(d,p)
The BH3 optimisation (6-31G(d,p)) file is linked to here
| Q | A |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -26.61532363 ±0.003808780 a.u. |
| Gradient | 0.00000235 a.u. |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 47.0 seconds |
The corresponding log book:
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000019 0.001800 YES RMS Displacement 0.000012 0.001200 YES Predicted change in Energy=-1.305135D-10 Optimization completed.
The optimised B-H bond distance: 1.19 ±0.01 Å
the optimised H-B-H bond angle: 30.0ο ±0.01ο
The total energy for 3-21G optimised structure: -26.46226338 ±0.003808780 a.u.
The total energy for 6-31G(d,p) optimised structure: -26.61532363 ±0.003808780 a.u.
Day Two
GaBr3
Link to "D-space": DOI:10042/25222
| Q | A |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -9638.24603597 ±0.003808780 a.u. |
| Gradient | 0.00006765 a.u. |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 52.5 seconds |
The corresponding log book:
Item Value Threshold Converged? Maximum Force 0.000135 0.000450 YES RMS Force 0.000089 0.000300 YES Maximum Displacement 0.000814 0.001800 YES RMS Displacement 0.000533 0.001200 YES Predicted change in Energy=-1.781658D-07 Optimization completed.
The optimised Ga-Br bond distance: 2.26 ±0.01 Å
The optimised Br-Ga-Br bond angle: 120.0ο ±0.1ο
The literature value of Ga-Br bond distance is found to be 2.249 Å (method ED) [1]
The difference between experimental value of Ga-Br bond distance and literature value of Ga-Br bond distance is 0.01 Å which is quite small. One of the reasons accounting for the difference is that two different calculation methods are used. Also, the chosen basis set for this experiment is not the most accurate one, therefore the result is not expected to have a high accuracy.
BBr3
The BBr3 optimisation file is linked to here
| Q | A |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -64.43645296 ±0.003808780 a.u. |
| Gradient | 0.00000382 a.u. |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 16.5 seconds |
The corresponding "Item" table:
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES Predicted change in Energy=-4.026916D-10 Optimization completed..
The optimised B-Br bond distance: 1.93 ±0.01 Å
The optimised Br-B-Br bond angle: 120.0ο ±0.1ο
Analysis
| Molecule | Bond Distance /Å |
|---|---|
| BH3 | 1.19232 |
| GaBr3 | 2.26286 |
| BBr3 | 1.93396 |
Bond becomes longer when the H atom from B-H bond is replaced by the Br atom. The B-Br bond is 0.74164 Å longer than the B-H bond. Also, the BBr3 has an energy of -64.43645296 a.u. which means the energy of the molecule has increased, comparing to the original molecule (BH3) which has an energy of -26.61532363 a.u..
In terms of similarity, both H and Br atoms have one unpaired electron in their valence shells and they are both X-type ligands. However, the Br atom have a electron configuration of [Ar] 3d104s24p5, which is much larger in size than the H atom (1S1). It is also reflected in the increased bond length. In addition to that, the electronegativity of Br atom is higher than that of H atom, leading to a more polarised B orbital, which makes bond longer.
Both B atom and Ga atom are from group 13, having a valence electron number of 3. But the valence orbitals of Ga are 4s4p while the valence orbitals of B are 2s2p.
When the central element of BBr3 is changed from B atom to Ga atom, the bond length is increased from 1.93396 Å of B-Br to 2.26286 Å of Ga-Br. This is because the Ga atom is larger in size. However, the energy is increased significantly from -26.61532363 a.u. to -9638.24603597 a.u.. The reason for that is the poor orbital overlap between Ga valence orbitals (4s4p) and Br valence orbitals(4s4p). The 4s and 4p orbitals are more diffuse than the 2s and 2p orbitals of B atom, resulting in a weaker Ga-Br covalent bond and higher in energy.
In some cases, no bonds are shown on the gaussview, but it does not mean there is no bond. Gaussview only displays bonds when the length of the bond is within the range of pre-defined value. [2] A chemical bond is the attraction between atoms that holds two or more atoms together to form a new chemical substance. The attraction exists between two opposite charges (electrostatic attraction). Chemical bonds are divided into various categories based on the strength of the interaction. For example, an ionic bond implies a strong interaction between atoms, usually with an electronegativity difference larger than 1.7.
Day Three
BH3 Frequency Analysis
The BH3 frequency analysis file is linked to here
| Q | A |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -26.61532363 ±0.003808780 a.u. |
| Gradient | 0.00000237 a.u. |
| Imaginary Frequency | 0 |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 16.0 seconds |
The corresponding "Item" book:
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000019 0.001800 YES RMS Displacement 0.000009 0.001200 YES Predicted change in Energy=-1.323376D-10 Optimization completed.
Low frequencies --- -0.9033 -0.7343 -0.0054 6.7375 12.2491 12.2824 Low frequencies --- 1163.0003 1213.1853 1213.1880
IR spectrum of BH3, click to enlarge:
As it can be seen from the diagram, only three peaks are shown on the IR spectrum while there are six vibrations in total according to the calculation. The forth vibration is totally symmetric (A1'), therefore it is IR inactive and no corresponding peak will appears on IR spectrum. The second and third vibrations have the same vibrational frequencies at 1213.19 cm-1. Thus, the two peaks are overlapped and only one peak is shown on the IR spectrum. It is the same for the fifth and sixth vibrations, which share the vibrational frequencies at 2715.43 cm-1.
GaBr3 Frequency Analysis
Link to "D-space": DOI:10042/25339
| Q | A |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -9638.24603597 ±0.003808780 a.u. |
| Gradient | 0.00006761 a.u. |
| Imaginary Frequency | 0 |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 39.8 seconds |
The corresponding "Item" book:
Item Value Threshold Converged?
Maximum Force 0.000135 0.000450 YES
RMS Force 0.000068 0.000300 YES
Maximum Displacement 0.000813 0.001800 YES
RMS Displacement 0.000406 0.001200 YES
Predicted change in Energy=-1.648213D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -5.2266 -3.2556 -3.2556 0.0104 0.0140 0.0222 Low frequencies --- 86.7293 86.7295 119.8002
The lowest "real" mode is the vibration at 0.0104
Click on the image for an enlarged IR spectrum:

| Molecule | Vibrational Frequency / ±10 cm-1 | Mode |
|---|---|---|
| BH3 | 1163 | A2" |
| BH3 | 1213 | E' |
| BH3 | 1213 | E' |
| BH3 | 2582 | A1' |
| BH3 | 2715 | E' |
| BH3 | 2715 | E' |
| GaBr3 | 87 | E' |
| GaBr3 | 87 | E' |
| GaBr3 | 120 | A2" |
| GaBr3 | 236 | A1' |
| GaBr3 | 366 | E' |
| GaBr3 | 366 | E' |
It is clear that there is a large difference between vibrational frequencies of BH3 and vibrational frequencies of GaBr3. Since all the bonds are single bonds, a larger value of vibrational frequency corresponds to a stronger covalent bond. In this case, the bond between B-H is stronger than the bond between Ga-Br. B-H bond is stronger because of the better 1s-2p orbital overlap. 1s orbital is the smallest orbital and therefore it has a stronger overlap with 2p orbital than another p orbital. Both of Ga and Br atoms are in the 4th period corresponding to a larger (more diffuse) valence orbital (4p), which makes their orbital overlap weaker than the overlap between 1s and 2p orbitals.
It should be noticed that both of the IR spectra displays three peaks while there are six vibrational modes in each molecule. Corresponding explanation for GaBr3 is the same as for BH3.
For both spectra, A2" and E' modes lie closely together and A1' and E' modes lie closely together. However the A1' and E' modes have a higher vibrational frequencies than that of A2" and E' modes. By observing vibrations, it is clear that there are no bond stretching for A2" and E' modes, bond distance remains the same for those three vibrations (e.g. rocking). Yet for A1' and E' modes, there are bond stretching in all three vibrations. It implies that vibrational frequencies tend to be higher with the vibration mode with bond stretching.
Why must you use the same method and basis set for both the optimisation and frequency analysis calculations?
Different method and basis set represent different approximations and accuracies in solving Schrodinger equation, therefore the results cannot be compared if different method and basis set are chosen for the calculation.
What is the purpose of carrying out a frequency analysis?
Frequency analysis can help to confirm if the minimum structure is achieved. It corresponds to second derivative of the potential energy surface. Thus, positive frequencies stand for the minimum energy, negative frequencies represent the failure to achieve an optimisation. provides the IR and Raman modes to compare with experiment.
What do the "Low frequencies" represent?
Low frequencies represent the vibrations with minimum energy
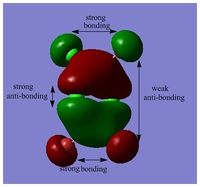
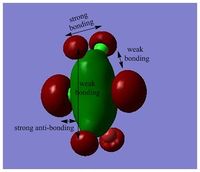
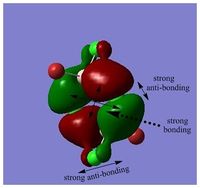
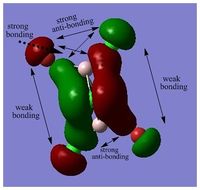
MOs of BH3
Link to "D-space": DOI:10042/25345
| Q | A |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -26.61532363 ±0.003808780 a.u. |
| Dipole Moment | 0.00 ±0.01 Debye |
| Point Group | D3h |
| Job Time | 0 days 0 hours 0 minutes 6.2 seconds |
LCAO MO is a approximation developed for certain types of chemical systems, it might not be applicable to the other chemical systems. The choice of basis set can be inadequate and would result in a poor accuracy. However, as an inexpensive method, MOs and population analysis are usually easy to interpret. [3]
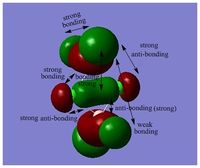
MO diagram of BH3 can be found here:
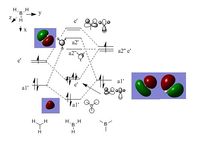
NH3
| Calculation Type | FOPT | FREQ | SP |
|---|---|---|---|
| Calculation Method | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 0 | ||
| Spin | Singlet | Singlet | Singlet |
| Final Energy in Atomic Units / ±0.003808780 a.u. | -56.55776856 | -56.55776856 | -56.55776856 |
| Gradient / a.u. | 0.00000885 | 0.00000891 | |
| Imaginary Frequency | |||
| Dipole Moment / ±0.01 Debye | 1.85 | 1.85 | 1.85 |
| Point Group | C1 | C1 | C1 |
| Job cpu Time | 0 days 0 hours 1 minutes 5.0 seconds | 0 days 0 hours 0 minutes 22.0 seconds | 0 days 0 hours 0 minutes 6.5 seconds |
| log file Link | Optimisation here | Frequency here | MO here |
"Item" book of optimisation file:
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000079 0.001800 YES RMS Displacement 0.000053 0.001200 YES Predicted change in Energy=-1.629716D-09 Optimization completed.
"Item" book of frequency file:
Item Value Threshold Converged? Maximum Force 0.000021 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000077 0.001800 YES RMS Displacement 0.000039 0.001200 YES Predicted change in Energy=-1.610514D-09 Optimization completed.
Low frequencies:
Low frequencies --- -30.6683 -0.0015 0.0008 0.0012 20.3158 28.3272 Low frequencies --- 1089.5567 1694.1245 1694.1869
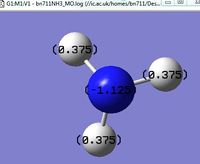
Image of charge distribution (-1.0 to +1.0),click on the image to enlarge.
Image of the specific NBO charges. As it can be seen from the graph, the nitrogen atom has a charge of -1.125 and each hydrogen atom has a charge of 0.375.
Ammonia-Borane
The NH3BH3 optimisation file is linked to here The NH3BH3 frequency file is linked to here
| Q | A |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -83.22469007 ±0.003808780 a.u. |
| Gradient | 0.00006839 a.u. |
| Dipole Moment | 5.56 ±0.01 Debye |
| Point Group | C1 |
| Job Time | 0 days 0 hours 1 minutes 6.5 seconds |
"Item" book of NH3BH3 optimisation file
Item Value Threshold Converged? Maximum Force 0.000139 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000771 0.001800 YES RMS Displacement 0.000338 0.001200 YES Predicted change in Energy=-2.028054D-07 Optimization completed.
| Q | A |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -83.22469014 ±0.003808780 a.u. |
| Gradient | 0.00006833 a.u. |
| Imaginary Frequency | 0 |
| Dipole Moment | 5.56 ±0.01 Debye |
| Point Group | C1 |
| Job Time | 0 days 0 hours 0 minutes 48.7 seconds |
Low frequencies --- -0.0011 -0.0008 0.0002 19.1416 23.8752 42.9246
Low frequencies --- 266.5875 632.3849 639.5915
Diagonal vibrational polarizability:
5.0198170 2.5459889 2.5468169
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 266.5824 632.3849 639.5914
Red. masses -- 1.0078 4.9926 1.0452
Frc consts -- 0.0422 1.1764 0.2519
IR Inten -- 0.0000 13.9835 3.5494
E(BH3)= -26.61532363 ±0.003808780 a.u.
E(NH3)= -56.55776856 ±0.003808780 a.u.
E(NH3BH3)= -83.22469007 ±0.003808780a.u.
ΔE=E(NH3BH3)-[E(NH3+E(BH3)]= -0.05159788 ±0.003808780 a.u. = -140 ±10 kJ/mol
Mini Project
Optimisation
| Name | Isomer 1 | Isomer 2 | Isomer 3 | Isomer 4 |
|---|---|---|---|---|
| Image | 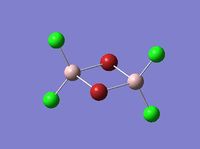 |
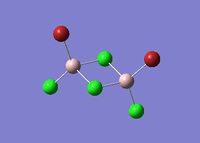 |
 |
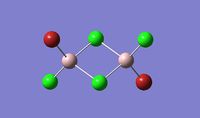 |
| Calculation Type | FOPT | FOPT | FOPT | FOPT |
| Calculation Method | RB3LYP | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | Gen | Gen | Gen | Gen |
| Charge | 0 | 0 | 0 | 0 |
| Spin | Singlet | Singlet | Singlet | Singlet |
| Final Energy in Atomic Units / ±0.003808780 a.u. | -2352.40630798 | -2352.41626681 | -2352.41109945 | -2352.41631606 |
| Gradient / a.u. | 0.00000079 | 0.00000124 | 0.00000491 | 0.00001799 |
| Imaginary Frequency | ||||
| Dipole Moment / ±0.01 Debye | 0.00 | 0.17 | 0.14 | 0.01 |
| Point Group | C2v | C2v | C1 | CS |
| Job cpu Time | 0 days 0 hours 3 minutes 9.9 seconds | 0 days 0 hours 2 minutes 41.8 seconds | 0 days 0 hours 4 minutes 56.0 seconds | 0 days 0 hours 2 minutes 13.7 seconds |
| D-Space Link | DOI:10042/25574 | DOI:10042/25575 | DOI:10042/25573 | DOI:10042/25717 |
E(isomer1)= -2352.40630798 a.u. = -6176240 ±10 kJ/mol
E(isomer2)= -2352.41626681 a.u. = -6176260 ±10 kJ/mol
E(isomer3)= -2352.41109945 a.u. = -6176250 ±10 kJ/mol
E(isomer4)= -2352.41632893 a.u. = -6176270 ±10 kJ/mol
Isomer 4 possesses the lowest energy among all four isomers, therefore it is set to be the relative energy. The difference between the energy of each isomer and the relative energy is taken and the following table is obtained.
| Highest Energy |  |
 |
 |
 |
Lowest Energy |
|---|
From the table above, it can be seen that there's a small energy difference (0.12931 kJ/mol) between two isomers with none of the bromine atoms in the bridging positions. However, when one of the bridging chlorine atom is replaced by a bromine atom, the energy is increased by 13.69621 kJ/mol. And when both of the bridging chlorine atoms are substituted by bromine atoms, energy is increased by 26.27622 kJ/mol, which is twice the increased energy when only one bromine atom is in the bridging position. This indicates that the energy of Al2Br2Cl4 is highly dependent on the number of bridging bromine atoms. Cl atom and Al atom are in the same period, therefore they have a good overlap between their valence orbitals (3p), resulting in a lower energy and more stable covalent bond. Yet, Br atom has valence orbitals of 4p which is a poor overlap with 3p orbitals of Al atom, leading to a higher energy and less stable covalent bond.
Overall, bromine atoms do not favour a bridging position in Al2Cl4Br2 compared to chlorine atoms, isomer 4 which has two bridging Cl atoms is the most stable isomer.
Optimisation analysis is carried out on AlBrCl2, the following result is obtained.
| Image | 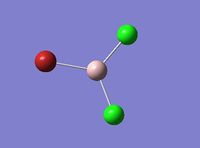 |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units / ±0.003808780 a.u. | -1176.19013679 |
| Gradient / a.u. | 0.00004196 |
| Imaginary Frequency | |
| Dipole Moment / ±0.01 Debye | 0.11 |
| Point Group | C2v |
| Job cpu Time | 0 days 0 hours 0 minutes 36.8 seconds |
| D-Space Link | DOI:10042/25697 |
"Item" book of AlBrCl2 Optimisation
Item Value Threshold Converged? Maximum Force 0.000136 0.000450 YES RMS Force 0.000073 0.000300 YES Maximum Displacement 0.000681 0.001800 YES RMS Displacement 0.000497 0.001200 YES Predicted change in Energy=-7.984418D-08 Optimization completed.
E(AlBrCl2)= -1176.19013679 ±0.003808780 a.u. = -3088090 ±10 kJ/mol
Dissociation Energy = 2E(AlBrCl2) - E(isomer4)= 2*-3088087.20414 - (-6176269.03782)= 90 ±10 kJ/mol
The dissociation energy shows a positive value which means that the dimer has a lower energy than two monomers. Therefore the product (dimer) is more stable than isolated monomers.
Frequency Analysis
| Name | Isomer 1 | Isomer 2 | Isomer 3 | Isomer 4 |
|---|---|---|---|---|
| Image |  |
 |
 |
 |
| Calculation Type | FREQ | FREQ | FREQ | FREQ |
| Calculation Method | RB3LYP | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | Gen | Gen | Gen | Gen |
| Charge | 0 | 0 | 0 | 0 |
| Spin | Singlet | Singlet | Singlet | Singlet |
| Final Energy in Atomic Units / ±0.003808780 a.u. | -2352.40630798 | -2352.41626681 | -2352.41109945 | -2352.41631606 |
| Gradient / a.u. | 0.00000076 | 0.00000127 | 0.00000490 | 0.00001799 |
| Imaginary Frequency | 0 | 0 | 0 | 0 |
| Dipole Moment / ±0.01 Debye | 0.00 | 0.17 | 0.14 | 0.01 |
| Point Group | C2v | C2v | C1 | CS |
| Job cpu Time | 0 days 0 hours 1 minutes 18.5 seconds | 0 days 0 hours 1 minutes 35.3 seconds | 0 days 0 hours 3 minutes 17.8 seconds | 0 days 0 hours 2 minutes 5.7 seconds |
| IR Spectrum |  |
 |
 |
 |
| D-Space Link | DOI:10042/25647 | DOI:10042/25648 | DOI:10042/25649 | DOI:10042/25718 |
Different isomers have different symmetries, which implies that different number of vibrations and type of modes are expected. Some vibrational modes display no change in dipole moment, which makes them IR inactive. An IR active vibrational mode requires at least one change in dipole [4]. Otherwise it would not be detected by IR spectrometer.
MO
| Q | A |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | GEN |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units | -2352.41631606 ±0.003808780 a.u. |
| Gradient | 0.00000000 a.u. |
| Dipole Moment | 0.01 ±0.01 Debye |
| Point Group | CS |
| Job Time | 0 days 0 hours 0 minutes 19.6 seconds |
| D-Space Link | DOI:10042/25730 |
Further Study
There is a possibility should be considered which is the presents of the fifth isomer. Al2Cl4Br2 could undergo interchange, resulting in the attachment of both bromine atoms to the same aluminum atom on terminal positions. The fifth isomer is being optimised and the following summary is obtained.
| Image |  |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Charge | 0 |
| Spin | Singlet |
| Final Energy in Atomic Units / ±0.003808780 a.u. | -2352.41632893 |
| Gradient / a.u. | 0.00000939 |
| Imaginary Frequency | |
| Dipole Moment / ±0.01 Debye | 0.19 |
| Point Group | CS |
| Job cpu Time | 0 days 0 hours 3 minutes 13.2 seconds |
| D-Space Link | DOI:10042/25656 |
"Item" book of isomer 5 Optimisation
Item Value Threshold Converged? Maximum Force 0.000021 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000285 0.001800 YES RMS Displacement 0.000115 0.001200 YES Predicted change in Energy=-7.918666D-09 Optimization completed.
When it is compared to the other four isomers, isomer 5 shows a lower energy than the energies of other four isomers, meaning it is more stable. Therefore, based on the calculations, there is a high chance of the existence of isomer 5.
References
- ↑ W. M. Haynes, D. R. Lide and T. J. Bruno, CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data, 2012, 93, 9–23.
- ↑ Hunt Research Group, Understanding optimisation part a., http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year3/3a_understand_opt.html
- ↑ B. E. Bursten, Some comments on approximate LCAO molecular orbital theory in organometallic chemistry: Getting more by doing less?, Pure & Appl. Chem., 1991, 63, 841.
- ↑ J. D. Paula, P. Atkins, Elements of physical chemistry, 2009, 5, 459