Rep:Mod:Mywiki
Ammonia
Calculations were carried out using the programme "Gaussian".
Summary of Results
| Calculation method: | RB3LYP |
| Basis Set: | 6-31G(d,p) |
| Final Energy E(RB3LYP): | -56.55776873 a.u. |
| RMS Gradient Norm: | 0.00000485 a.u. |
| Point Group: | C3V |
Item Table from log file
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
It can be seen that all the items have converged. This shows that the optimisation of the molecule was successful.
Bond angle and Bond Length
The H-N-H bond angle is 105.74115 degrees. The N-H bond length is 1.01798 Angstrom.
Ammonia molecule |
View the log file here:
File:MALA NH3 OPTF POP.LOG
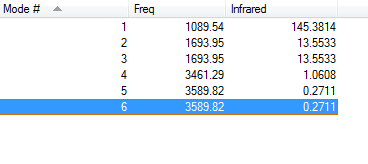
Vibrations of NH3 molecule in IR spectrum
From the 3N-6 rule, 3 vibrational modes are expected. Modes 2 and 3 (1693.95 cm-1), and 5 and 6 (3589.82 cm-1) are degenerate. Modes 1 and 2(or 3) are bending vibrations, while Modes 4 and 5 (or 6) are stretching vibrations. Mode 4 is highly symmetric (as the dipole doesn't change) Mode 1 is the "umbrella" mode. 3 bands would be expected to show up in an experimental spectrum of gaseous ammonia.
Since Nitrogen is more electronegative than Hydrogen, you'd expect N to have a negative charge, and H to have a positive charge. This is confirmed by the calculations which give N having a charge of -1.125 and H 0.375.
Nitrogen
Summary of Results
| Calculation method: | RB3LYP |
| Basis Set: | 6-31G(d,p) |
| Final Energy E(RB3LYP): | -109.52412868 a.u. |
| RMS Gradient Norm: | 0.00000365 a.u. |
| Point Group: | D∞H |
Item Table from log file
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Nitrogen molecule |
View the log file here:
File:MALA N2 OPTF POP.LOG
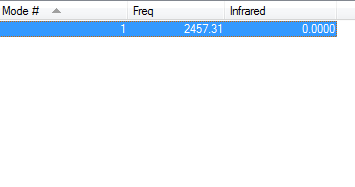
Vibrations of N2 in IR spectrum
For linear molecules, the 3N-5 rule is followed. This predicts the number of vibrational modes in nitrogen to be 1- which is in agreement with our calculation.
There is one stretching vibration at 2457.31 cm-1. However, this does not show up in the infrared spectrum as N-N has no net dipole moment.
Bond Length
The bond length of Nitrogen is 1.10550 Angstrom.
Hydrogen
Summary of Results
| Calculation method: | RB3LYP |
| Basis Set: | 6-31G(d,p) |
| Final Energy E(RB3LYP): | -1.17853936 a.u. |
| RMS Gradient Norm: | 0.00000017 a.u. |
| Point Group: | D∞H |
Item Table from log file
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Hydrogen molecule |
View the log file here:
File:H2 mp.LOG
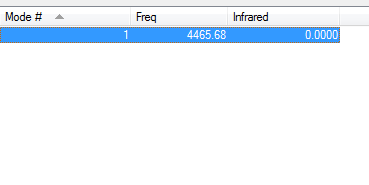
Vibrations of H2 in IR spectrum
Since Hydrogen is linear, the 3N-5 rule is followed and 1 vibrational mode is predicted. This is the stretching frequency at 4465.68 cm-1. This is a very high frequency since hydrogen atoms and small and thus the hydrogen bond is strong.
Like nitrogen, hydrogen has no net dipole moment and therefore has zero intensity in the IR spectrum.
Bond Length
The H-H bond length is 0.74279 Angstrom. It is a linear molecule, and like nitrogen, has no net dipole since it is symmetric.
Haber-Bosch Reaction Energy
| E(NH3) | -56.55776873 a.u. |
| 2*E(NH3) | -113.1155375 a.u. |
| E(N2) | -109.52412868 a.u. |
| E(H2) | -1.17853936 a.u. |
| 3*E(H2) | -3.53561808 a.u. |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.0557907 a.u. = -146.5 kJ mol-1 |
The enthalpy of formation of ammonia is -45.73112 kJ mol-1- from this information, the formation of 2 moles of ammonia would be 91.46 kJ mol-1.[1] [1] This is smaller than the Gaussian value of -146.5 kJ mol-1- this is due to the fact that the conditions used in Gaussian are different from actual experimental conditions. Since the reaction is exothermic, it can be seen that the formation of ammonia is favoured, over the formation of its gaseous reactants (N2 and H2).
Chlorine
Summary of Results
| Calculation method: | RB3LYP |
| Basis Set: | 6-31G(d,p) |
| Final Energy E(RB3LYP): | -920.34987886 a.u. |
| RMS Gradient Norm: | 0.00002948 a.u. |
| Point Group: | D∞H |
Item Table from log file
Item Value Threshold Converged? Maximum Force 0.000051 0.000450 YES RMS Force 0.000051 0.000300 YES Maximum Displacement 0.000143 0.001800 YES RMS Displacement 0.000202 0.001200 YES
The Cl-Cl bond length is 2.04176 Angstrom, and the molecule is linear.
Chlorine molecule |
View the log file here:
File:MP CL2 OPTF POP.LOG
Vibrations of Cl2 in IR spectrum
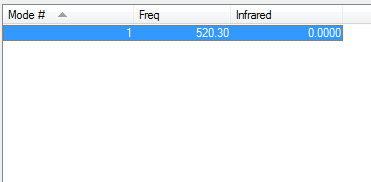
Chlorine follows the 3N-5 rule and 1 vibrational mode is predicted. This is the stretching vibration at 520.30 cm-1. This frequency is small since Chlorine atoms are big and the bond length is large.
Like the other diatomic gases, chlorine has no net dipole moment.
Molecular Orbitals in Chlorine
Mixing of Molecular orbitals:
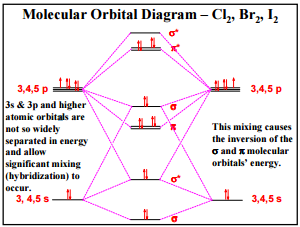
Figure 1 taken from http://web.utk.edu/~adcock00/g531ch17.pdf
As seen in the Molecular Orbital diagram, mixing occurs between bonding s and p σ orbitals when the two bonding orbitals are close in energy to each other. This is done to lower the energy of the lower energy sσ bonding orbital and to raise the energy of the higher energy pσ bonding orbital.[2] This is why one of the sigma bonding orbitals (formed from 3pz overlap along the internuclear axis) is higher in energy than the pi bonding orbitals (formed by 3px orbitals and 3py orbitals). Mixing allows greater orbital overlap and makes the formation of the molecule more exothermic.[2] [2]
References
- ↑ King, L. (1972). The enthalpies of ammonia ’ and formation, (January 1968), 675–683. (King, 1972)
- ↑ Inagaki, S., Fujimoto, H., & Fukui, K. (1976). Orbital mixing rule. Journal of the American Chemical Society, 98(14), 4054–4061. http://doi.org/10.1021/ja00430a006