Rep:Mod:Molecular modelling 2
Ammonia
Summary Information
Here are the measurements for ammonia when optimised in GaussView
| Optimisation measurements | Values |
|---|---|
| bond length | 1.01798 Å |
| bond angle | 105.741° |
| calculation method | B3LYP |
| basis set | 6-31G(d.p) |
| RMS gradient | 0.0000000485 (a.u) |
| final energy | -56.5578 (a.u) |
| point group | C3v |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
This table shows the values at equilibrium when all the forces are at zero. Calculations show a non zero number due to rounding errors.
Optimised ammonia |
Optimisation file for NH3 is linked here
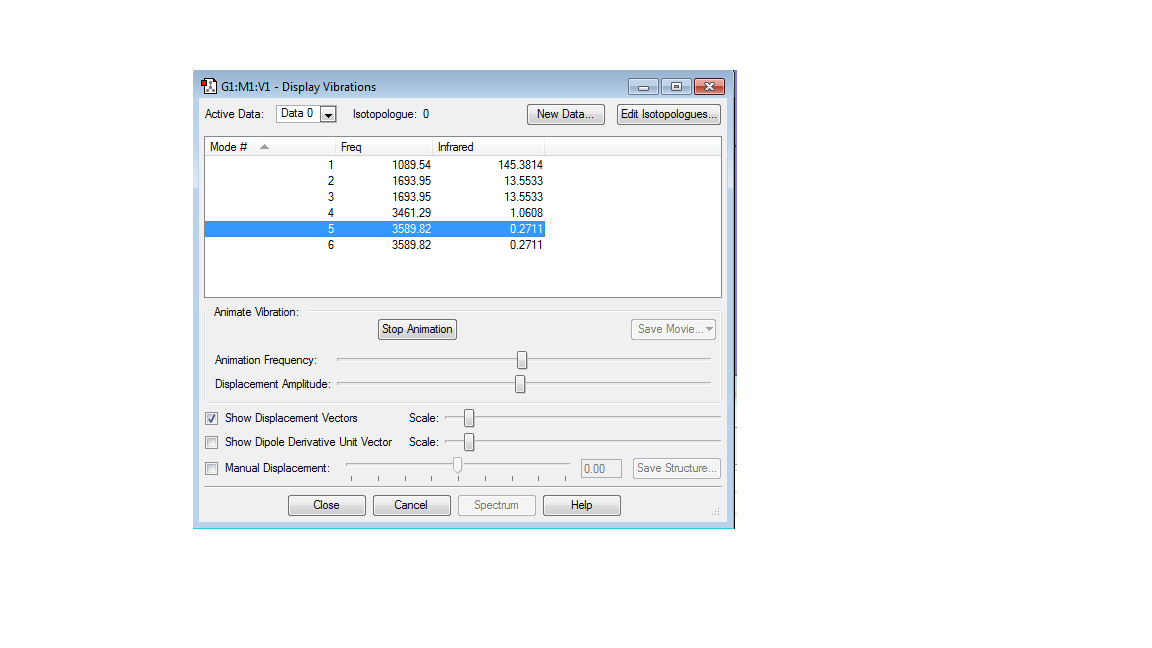
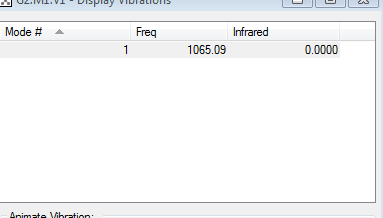
Vibrational Modes
Ammonia can absorb infrared, IR causes a dipole moment making ammonia IR active.
Modes 2/3 and 5/6 are degenerate, table shows they have the same value of energy. In the spectrum only visible modes are 1 and 2/3, however modes 4/5 and 6 are small values only visible by zooming in.
Table to show the types of vibrations associated with each mode:
| Modes | Vibrations |
|---|---|
| 1 | bending, symmetric, also known as the umbrella mode |
| 2 | bending |
| 3 | bending |
| 4 | stretch, is highly symmetric |
| 5 | stretch |
| 6 | stretch |
All other vibrations are asymmetric. In an experimental spectrum yo would see two peak intensities for mode 1 and 2/3. The values of the other modes relative to 1 and 2/3 are too small to be shown.
Charge Distribution

Nitrogen is the more electronegative species and so is expected to have negative dipole and therefore hydrogen is expected to have a positive dipole. Total charge is neutral the sum of all charges is 0.
Nitrogen
Summary Information
Values obtained for optimised nitrogen
| Optimisation measurements | Values |
|---|---|
| bond length | 1.10550Å |
| bond angle | 180° |
| calculation method | B3LYP |
| basis set | 6-31G(d.p) |
| RMS gradient | 0.0000006 (a.u) |
| final energy | -109.5241 (a.u) |
| point group | D*H |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.400996D-13
Optimised Nitrogen |
Optimisation file is linked here
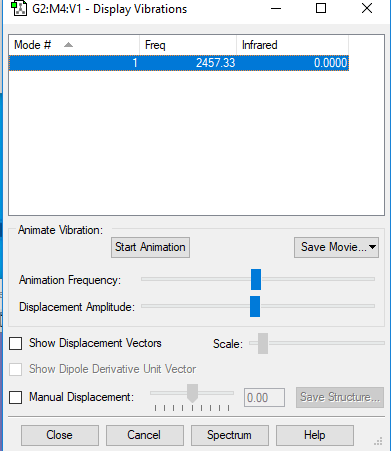
Vibrational Modes
NO dipole moment therefore not IR active. No difference in charge because it is a diatomic molecule so both have the same electronegativity.
Hydrogen
Summary Information
Optimisation values for H2
| Optimisation measurements | Values |
|---|---|
| bond length | 0.74279 Å |
| bond angle | 180° |
| calculation method | RB3LYP |
| basis set | 6-31G(d.p) |
| RMS gradient | -1.17853936 (a.u) |
| final energy | 0.0000017 (a.u) |
| point group | D*H |
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
Optimised Hydrogen |
Optimisation file is linked here
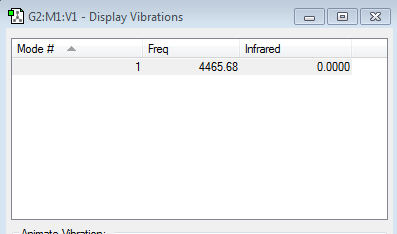
Vibrational Modes
Symmetric vibrations, no dipole moment occurs therefore is IR inactive.
Hydrogen also has no difference in charge distribution.
Haber-Bosch Process
N2+3H2→2NH3 This is the process used to make ammonia, using the values of energy calculated before we can create a Hess's cycle to work out the enthalpy of reaction.
E(NH3)= -56.5578 2*E(NH3)= -113.1156 E(H2)= -1.17853936 E(N2)= -109.5241 3*E(H2)= 3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074 ΔE= -146.650 kj/mol
The standard value for the standard enthalpy of formation of ammonia is -45.910 kjmol-1 [1] standard enthalpy changes form only one mole of product, therefore we will to multiply this value to compare to the Haber-Bosch process.
Using the standard enthalpy change, value for the process should be -91.82 kjmol-1. Differences in values are due to the optimisation as it takes into consideration the molecule at 0 K also standard enthalpy deals with moles while the optimisation only works with 1 molecule of ammonia.
Cyanide
Summary Information
| Optimisation measurements | Values |
|---|---|
| bond length | 1.18409Å |
| bond angle | 180° |
| calculation method | RB3LYP |
| basis set | 6-31G(d.p) |
| RMS gradient | -92.82453153 (a.u) |
| final energy | 0.00000704 (a.u) |
| point group | C*V |
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Predicted change in Energy=-6.650395D-11
Optimization completed.
-- Stationary point found.
Optimised Cyanide |
optimisation for cyanide is here
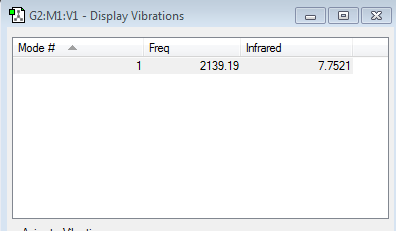
Vibrational Modes
Is IR active, molecule has a dipole moment. The spectrum for cyanide is large only has one vibration mode due to the 3n-5 rule, and since cyanide has a bond order of 3 rotation is restricted and therefore unable to bend. Only stretching occurs.
Charge Distribution
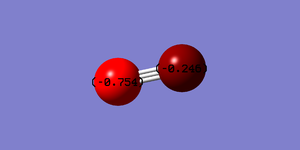
Total molecule is negative however there is more negative charge on the nitrogen. This is due to the nitrogen's greater electronegativity
Molecular Orbitals
Fluorine
Summary Information
| Optimisation measurements | Values |
|---|---|
| bond length | 1.40281Å |
| bond angle | 180° |
| calculation method | RB3LYP |
| basis set | 6-31G(d.p) |
| RMS gradient | 0.00007365(a.u) |
| final energy | -199.49825218 (a.u) |
| point group | D*H |
Item Value Threshold Converged?
Maximum Force 0.000128 0.000450 YES
RMS Force 0.000128 0.000300 YES
Maximum Displacement 0.000156 0.001800 YES
RMS Displacement 0.000221 0.001200 YES
Predicted change in Energy=-1.995024D-08
Optimization completed.
-- Stationary point found.
Optimised Fluorine |
Optimisation file is linked here
Vibrational Modes
Symmetric vibrations, no dipole moment occurs therefore is IR inactive.
Molecular Orbitals
| MOs | |
|---|---|
 |
σ* anti bonding orbital |
 |
π* anit bonding orbital |
 |
π bonding orbital |
 |
σ bonding orbital |
 |
bonding orbital |
References
- ↑ Sana, M.; Leroy, G.; Peeters, D.; Wilante, C. Journal of Molecular Structure, 1988 , vol. 164, p. 249 - 274