Rep:Mod:Me
NH3
The bond information of NH3
The following informations come from the structure of NH3 that has been optimised.
the N-H bond length
1.01865Å
the H-N-H bond angle
105.749°
Calculation summary
The table below is the calculation setting that has been used for optimizaing the molecule.
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy E(RB3LYP) | -56.55776762 a.u. |
| RMS gradient | 0.00032440 a.u. |
| point group | C3V |
The table below shows the final optimization of the NN3. The force is extremely close to 0 which means the attraction has the same magnitude as repulsion. This is the equilibrium point.
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000070 0.001800 YES RMS Displacement 0.000033 0.001200 YES Predicted change in Energy=-5.785208D-10 Optimization completed.
An image of NH3
NH3 |
The optimisation is link to the file: here
Bond Vibration in NH3
Display vibrations
•how many modes do you expect from the 3N-6 rule?
We expect 6 vibrational modes from the 3N-6 rule
•which modes are degenerate (ie have the same energy)?
From the figure attached above, it can be seen that mode 2 and mode 3 are degenerate (both have frequency of 1693.95HZ). Meanwhile, Mode 5 and mode 6 are degenerate as well (frequency=3589.82HZ).
•which modes are "bending" vibrations and which are "bond stretch" vibrations?
Mode 1, 2, 3 are bending vibrations. Mode 4, 5, 6 are bond stretching vibrations. Because bending requires less energy than stretching.
•which mode is highly symmetric?
Mode 4. The 3 H atoms are stretching in phase.
•one mode is known as the "umbrella" mode, which one is this?
Mode 1.
•how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
4. There are 2 peaks that are distinguishable. The other two peaks are too small to be seen , need to be zoomed in to be identified. This is because the vibration has a small change of dipole moment.
Charge on NH3
N:-1.125 c H:0.375 c Due to the electronegative of N is stronger than H, the charge on the N is more negative than H.
N2
The bond information of N2
the N-N bond length
1.09200Å
Calculation summary
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy E(RB3LYP) | -109.52412868 a.u. |
| RMS gradient | 0.00000060 a.u. |
| point group | D*H |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401060D-13 Optimization completed.
An image of N2
N2 |
The optimisation is link to the file: here
Bond Vibration in N2
Display vibrations
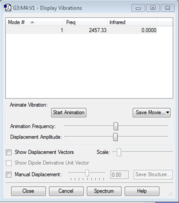
Since it is a linear molecule, there is only one stretching mode. Also N2 cannot be detected in infra red because there is no dipole in the molecule, when there is a stretch, there is no dipole change, so the infra-red value is 0.
Charge on N2
There are no charge on both of the atoms due to their identical electronegativity.
•how many modes do you expect from the 3N-5 rule?
We expect 1 vibrational modes from the 3N-5 rule due to the linear shape.
H2
The bond information of H2
the H-H bond length
0.74279Å
Calculation summary
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy E(RB3LYP) | -1.17853936 a.u. |
| RMS gradient | 0.00000004 a.u. |
| point group | D*H |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-6.835416D-15 Optimization completed.
An image of H2
H2 |
The optimisation is link to the file: here
Bond Vibration in H2
Display vibrations
Charge on H2
There is no charge on both of the atoms due to their identical electronegativity.
•how many modes do you expect from the 3N-5 rule?
We expect 1 vibrational modes from the 3N-5 rule due to the linear shape.
The energy of the reaction N2 + 3H2 -> 2NH3
•E(NH3)= -56.55776762
•2*E(NH3)= -113.11553524
•E(N2)= -109.52412868
•E(H2)=-1.17853936
•3*E(H2)= -3.53561808
•ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.05578848=-0.06 a.u(to 2 d.p)= -146.47kJ/mol(to 2 d.p)
[All energies of the molecules are recorded to 8 decimal places in a.u]
Product is more stable than reactants because the energy difference is negative. This means that the product is at lower energy level than reactants.[ΔE=E(product)-E(reactants)]
Cl2
The bond information of Cl2
The following informations come from the structure of Cl2 that has been optimised.
the Cl-Cl bond length
1.01865Å
Calculation summary
The table below is the calculation setting that has been used for optimizaing the molecule.
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy E(RB3LYP) | -56.55776762 a.u. |
| RMS gradient | 0.00032440 a.u. |
| point group | C3V |
The table below shows the final optimization of the NN3. The force is extremely close to 0 which means the attraction has the same magnitude as repulsion. This is the equilibrium point.
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000070 0.001800 YES RMS Displacement 0.000033 0.001200 YES Predicted change in Energy=-5.785208D-10 Optimization completed.
An image of Cl2
Cl2 |
The optimisation is link to the file: here
Bond Vibration in Cl2
Display vibrations

There is only one type of stretching mode as Cl2 is a linear molecule, it can only stretch but it cannot bend.
Cl2 cannot be detected in infra red because there is no dipole in the molecule, the two atoms have same electronegativity. When there is a stretch, there is no dipole change, so the infra-red value is 0.
Charge distrubution
There is no charge on both Cl atoms because they are identical atoms thus have same electronegativity. There is a covalent single bond in Cl2 as they share electons to become stable.
Molecular orbital


This Molecular orbital(MO)is an antibonding combination of two 2s atomic orbitals(AO) from each chlorine atom. The two orbitals with different colours indicates these two orbitals overlap out of phase. The picture on the right(the 4th MO) shows the bonding combination of two 2s AO of Cl. The identical colours shows that the overlapping is in phase.

This MO is a bonding combination of two 2Py AOs. The MO is occupied but because it is at a low energy level so it is not involve in chemical reactions. The 2Py combined MO has similar energy level as 2Pz
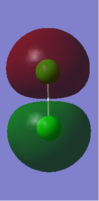
This MO is an antibonding combination of two 3S AOs. MO is occupied.
It forms the sigma* 3s MO.
Since this is an antibonding orbital, when it is occupied the bonding will be destabilised.
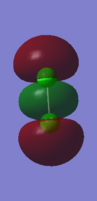
This figure shows an bonding combination of two 3Px AOs. The MO is occupied.
This MO has a high energy level compare with the 2P MO due to its outer position and thereofore it has better overlapping with the other 3P orbital.
Also, it is an bonding orbital, an occupied orbital will stable the bonding.


