Rep:Mod:Mb060691
Computational Lab Report
Part 1
Introduction
Cope rearrangement is the [3,3]-sigmatropic shift rearrangement of 1,5-dienes. The transition state at which Cope rearrangement proceeds has been a subject of debate, although the current accepted mechanism of Cope rearrangement is concerted through an aromatic transition state.
Optimizing Reactants and Products
There are 27 possible conformations for 1,5-hexadiene to adopt due to its three rotating carbon-carbon bonds. However, there are only 10 energetically unique conformers due to symmetry and enantiomeric relationships. However, in ab initio calculations, the activation energies and enthalpies uses the lowest energy (most stable) conformers as a reference reactant molecule. As a result, the structure of the conformer with the lowest energy must first be determined.
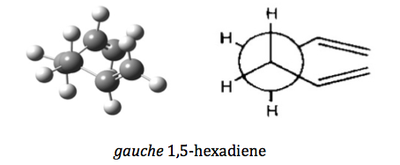
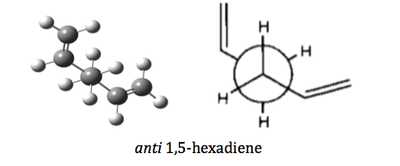
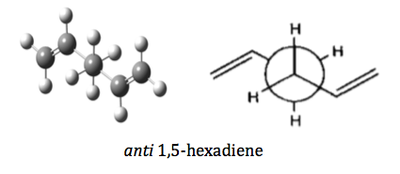
In this exercise, three conformers were optimized using three different levels of theories, namely semi-empirical/AM1, Hatree-Fock/3-21G and B3LYP/6-31G(d). The structure of the three conformers are given as below:
| Conformer | Structure | Point Group |
| gauche 1,5-hexadiene | C2 | |
| anti 1,5-hexadiene | C1 | |
| anti 1,5-hexadiene | Ci |
- Image of Newman Projection adapted from Rocque et all (see bibliography no.5)
Energy difference between anti and gauche 1,5-hexadiene conformers
The results are tabulated as below:
| Methods and Energies (a.u.) | |||
|---|---|---|---|
| Semi-empirical/AM1 | HF/3-21G | B3LYP/6-31G(d) | |
| Gauche 1,5-hexadiene (C2) | 0.02945203 | -231.69166700 | -234.61068822 |
| Anti 1,5-hexadiene (C1) | 0.02818095 | -231.69253506 | -234.61170276 |
| Anti 1,5-hexadiene (Ci) | 0.02816982 | -231.69260235 | -234.61180027 |
From the table above, it is apparent that the 1,5-hexadiene with the framework point group of Ci has the lowest energy.
However, it is interesting to note the difference in energy of the gauche conformer (C2) and anti conformer(C1. The difference in their respective energies generated using the three methods are tabulated below
| Difference in Energies (a.u.) | |||
|---|---|---|---|
| Semi-empirical/AM1 | HF/3-21G | B3LYP/6-31G(d) | |
| Difference in Energy | 0.00128221 | 0.00095650 | 0.00111205 |
| Average Difference Energies (a.u.) | 0.00111692 a.u. (2.931 kJ/mol or 0.7 kcal/mol) | ||
Despite using three different methods, the difference in energies between the gauche conformer (C2) and anti conformer(C1 is quite consistent, albeit quite small (about ten fold less than that of butadiene). It is postulated that the energy difference is due the steric repulsion of the two enyl groups in close proximity to one another. However, using higher level theories such as MP2 and CCSD(T), Rocque et al. determines that the energies of the 10 conformers are essentially degenerate. Their work also suggested that due to the lack of electron correlation in Hartree-Fock method, 1,5-hexadiene is treated too similarly to a butadiene which leads to poor results.
Geometry difference between anti and gauche 1,5-hexadiene conformers
Using different level of theories, the geometries of the molecule changes slightly. The difference in the structures are tabulated as below:

| Conformers and Methods | Bond length (Angstrom) | Bond angle (degrees) | ||||
| C1-C2 | C2-C3 | C3-C4 | a | b | ||
| Gauche | HF/3-21G | 1.31564 | 1.50832 | 1.55077 | 124.97390 | 112.04390 |
| B3LYP/6-31G(d) | 1.33309 | 1.50442 | 1.54815 | 125.39982 | 113.63603 | |
| Anti C1 | HF/3-21G | 1.31608 | 1.50888 | 1.55231 | 124.75456 | 111.37500 |
| B3LYP/6-31G(d) | 1.33347 | 1.50403 | 1.54771 | 125.24348 | 112.67108 | |
| Gauche | HF/3-21G | 1.31627 | 1.50908 | 1.55272 | 124.80187 | 111.35238 |
| B3LYP/6-31G(d) | 1.33351 | 1.50408 | 1.54808 | 125.28897 | 112.68043 | |
Rocque et al. also reported the bond length for C3-C4. Using CCSD(T) theory, his results are as below:
| Conformer | Bond length (Angstrom) |
| Gauche (C2) | 1.517 |
| Anti (C1) | 1.535 |
| Anti (Ci) | 1.535 |
Comparing both bond lengths, it is apparent in the deficiencies in the level of theories and methods used to run these calculations as compared to CCSD(T). While semi-empirical/AM1 method gave a good correlation with the C3-C4 bond length of the gauche conformation, it fails miserably with both the anti conformers. Meanwhile, although HF/3-21G and B3LYP/6-31G(d) is in close agreement with the C3-C4 bond length with CCSD(T), they have the tendency to overestimate the bond length, just as so with the energies.
Vibrational Frequencies of anti 1,5-hexadiene
Using a simple harmonic oscillator model, the vibrational frequencies of a molecule can be calculated by:

where: f = vibration frequency; k = force constant; m = reduced mass
It turns out that the force constant, k is equivalent to the second derivative of the potential energy curve.
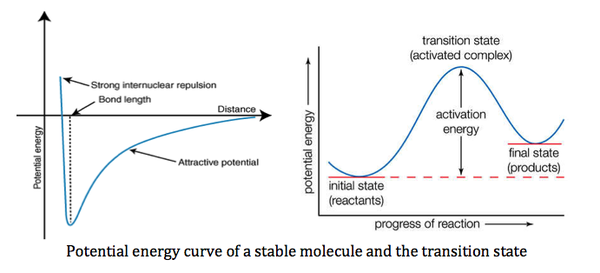
When a molecule is in its ground state (at equilibrium bond length), it has minimum energy. As the molecule vibrates and departs from its equilibrium bond length, the energy increases. Since the potential energy curve for all vibrational modes of the molecule increases, the second derivative would be positive, yielding a positive k and hence a positive and vibrational frequency.
However, in a transition state, the potential energy curve slopes downwards both forward and backwards from the transition state (maximum point). The second derivative would therefore yield a negative k and a negative vibrational frequency.
However, in this case, the anti-hexadiene (Ci) is being optimized hence no negative vibrational frequency should be observed and this is indeed the case.
Optimizing the “Chair” and “Boat” Transition Structures
The transition state at which Cope rearrangement proceeds has been postulated to go either via the chair transition state or the boat transition state. For the kinetically controlled Cope rearrangement reaction, the pathway with the lowest energy transition state would be the preferred mechanism.
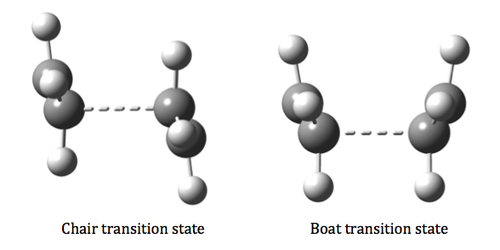
Using different methodology and 3 different level of theories (semi-empirical/AM1, HF/3-21G and B3LYP/6-31Gd), the bond breaking/forming distance of the transition state has been calculated. The vibration frequencies have been checked as well for a negative value to ensure that the structure obtained is indeed a transition state. For simplicity, only the energies of the transition states found by using HF/3-21G and B3LYP/6-31G(d) will be reported.
| B3LYP/6-31G(d) | ||||||
| Chair Transition State | Boat Transition State | |||||
|---|---|---|---|---|---|---|
| Normal | Freeze Coordinate | QST2 | Normal | Freeze Coordinate | QST2 | |
| Energy | -234.55698297 | - | - | -234.54309288 | - | -234.54309290 |
| Imaginary Frequencies | -565.4704 | - | - | -530.39 | - | -530.37 |
| HF/3-21G | ||||||
| Energy | -231.60280228 | -231.60068454 | -231.60280215 | -231.61932243 | -231.619322379 | - |
| Imaginary Frequencies | -839.5045 | -840.0922 | -839.5045 | -817.973 | -817.9719 | - |
The vibration modes of the negative vibrational frequencies are given as below:
| Transition State | Frontal view | Side View |
| Chair conformation | 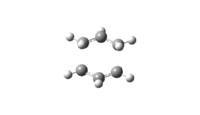
|
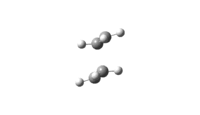
|
| Boat conformation | 
|
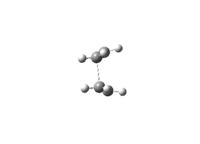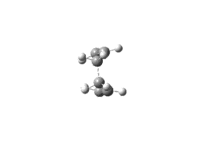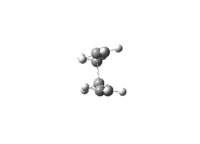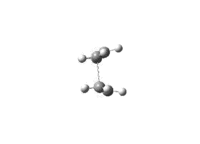
|
There bond lengths and bond angles of the transition state is characterized in the table below:
| B3LYP/6-31G(d) | ||||||
| Chair Transition State | Boat Transition State | |||||
|---|---|---|---|---|---|---|
| Normal | Freeze Coordinate | QST2 | Normal | Freeze Coordinate | QST2 | |
| C1-C2 | 1.40754 | - | - | 1.39319 | - | 1.39318 |
| C2-C3 | 1.40759 | - | - | 1.39324 | - | 1.39329 |
| a | 119.95231 | - | - | 122.26846 | - | 122.25770 |
| HF/3-21G | ||||||
| C1-C2 | 1.38930 | 1.38935 | - | 1.38155 | 1.38143 | 1.38126 |
| C2-C3 | 1.38925 | 1.38930 | - | 1.38155 | 1.38143 | 1.38126 |
| a | 120.50523 | 120.50305 | - | 121.66467 | 121.68511 | 121.67953 |
Intrinsic Reaction Coordinate
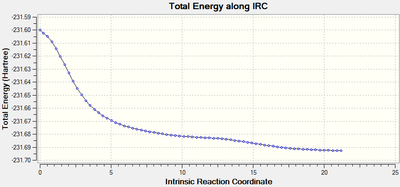
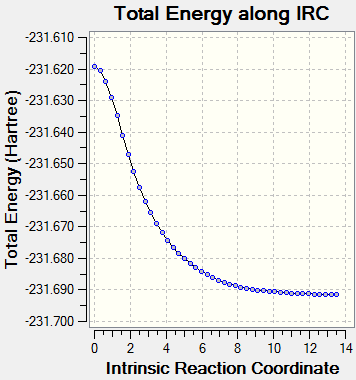
Although the transition state structure has been deduced, Intrinsic Reaction Method needs to run to determine which conformers the transition state will collapse into. Initially, the IRC method was run with N=50 points for both the boat and chair transition state, however, it was observed that a minima has not been achieved.
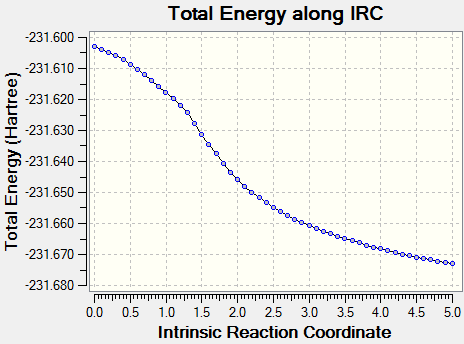
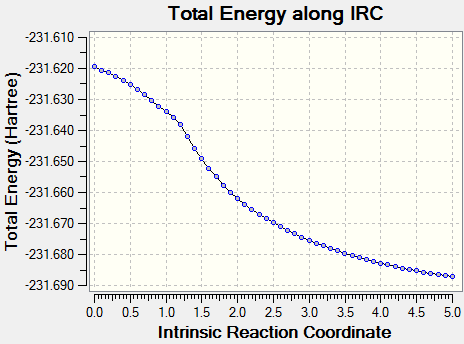
The problem was circumvented by increasing the number of points taken for the IRC i.e. N=100
Using the first method where the number of points taken is increased to N=100, that boat and chair transition has reached their respective minima. The minima of the chair transition state was found earlier, ending at N=64.
The final structure of the conformers produced by the chair and boat conformations are:
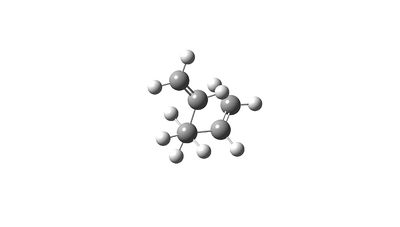
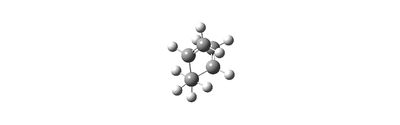
Summary of Part 1
Summary of energies (in hartree)
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||
| Chair TS | -231.619324 | -231.466700 | -231.461341 | -234.556983 | -234.414929 | -234.409008 |
| Boat TS | -231.600685 | -231.450926 | -231.445297 | -234.543093 | -234.402342 | -234.396008 |
| Reactant (anti2) | -231.692535 | -231.539506 | -231.532557 | -234.611703 | -234.469191 | -234.461794 |
Summary of activation energies (in kcal/mol)
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.686 | 44.689 | 34.050 | 33.124 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.585 | 54.756 | 41.948 | 41.281 | 44.7 ± 2.0 |
Part 2
Introduction
For a cycloaddition to occur, the orbitals on the molecules must have the right symmetry so that a positive overlap can occur. In this study, 1,3-butadiene and ethene is used to study the reaction of cycloaddition.

In this cycloaddition, the pi orbitals of the ethene will overlap with the pi orbitals of the diene (1,3-butadiene) to form new C-C bonds in a very stereopsecific fashion
The HOMO and LUMO of Butadiene
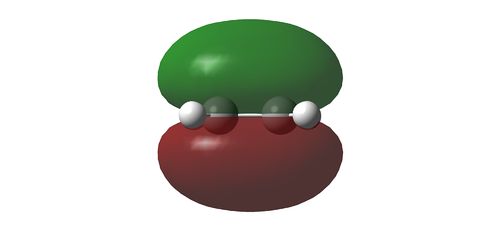
Using HF/3-21G, the HOMO and LUMO of the reactants in a cycloaddition is determined. With respect to a plane of symmetry perpendicular to the molecule, the HOMO of ethene and LUMO of the butadiene is symmetrical while the the HOMO of butadiene and the LUMO of ethene is assymmetrical. Hence, it is the HOMO-LUMO pair of the reactants that will interact with one another. The orbital pairs from the ethene and butadiene are illustrated as below
HOMO of 1,3-butadiene reacting with LUMO of ethene
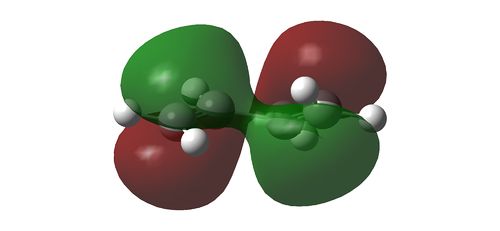
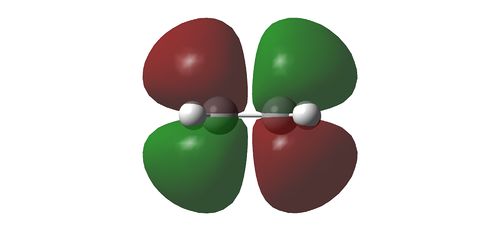
LUMO of 1,3-butadiene reacting with HOMO of ethene
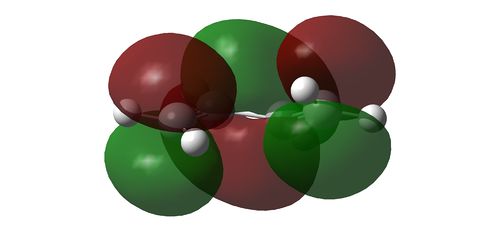
The Transition State of a Cycloaddition Reaction
To find the transition state of the cycloaddition reaction between 1,3-butadiene and ethene, the three level of theories (semi-empirical/AM1, HF/3-21G and B3LYP/6-31G(d)) is similarly employed.
The transition state of the cycloaddition between an ethene and a 1,3-butadiene is as below:
test molecule |
The findings are tabulated as below:
| Bond lengths and angles | Different Methods | ||
|---|---|---|---|
| Semi-empirical/AM1 | HF/3-21G | B3LYP/6-31G(d) | |
| Intra-reactant C-C bonds | |||
| C1-C2 | 1.38188 | 1.36998 | 1.38303 |
| C2-C3 | 1.39745 | 1.39448 | 1.40719 |
| C3-C4 | 1.38186 | 1.37000 | 1.38305 |
| C5-C6 | 1.38294 | 1.37585 | 1.38596 |
| a | 121.18427 | 121.47260 | 122.03073 |
| b | 121.18293 | 121.47293 | 122.03344 |
| Inter-reactant C-C bonds | |||
| C1-C5 | 2.11918 | 2.20960 | 2.27224 |
| C4-C6 | 2.11931 | 2.20956 | 2.27269 |
The typical bond length of an sp3 carbon-carbon is 1.54 angstrom, sp2 carbon-carbon bond is 1.47 angstrom while the Van der Waals radii of the carbon atom is 1.70 angstrom. However, in this instance, it is quite surprising to see that all the bond lengths calculated from Gaussian are shorter than the experimental values. This could be due to the fact that the level of theory used to calculate the bond lengths are insufficiently accurate.
In a normal instances, it is expected that the transition state bond length should be longer than normal. This is because as orbitals overlap during the cycloaddition, the electron density would flow from the diene to the dienophile. The loss of electron density would have lead to a decrease in bond order/bond strength.
The Cycloaddition Transition State Vibrational Frequencies
To ensure that it is indeed the transition state of the cycloaddition, the vibrational frequencies were checked and a negative frequency corresponding to -524.8453 was obtaine. The imaginary frequency is synchronous while the least positive vibration frequency (135.54) involves rocking from side to side in an asynchronous fashion. The vibrational mode of the imaginary frequency is illustrated as below:
Stretches that produce the imaginary frequency
Stretches that produce the least positive frequency

The Energies of the Cycloaddition Transition State
The energy of the transition state is given as below:
| Semi-empirical/AM1 | HF/3-21G | B3LYP/6-31G(d) | |
| Energy of Transition State (a.u.) | 0.11165468 | -231.60320853 | -234.54389654 |
The Molecular Orbitals of the Cycloaddition Transition State
The LUMO is unsymmetrical while the HOMO is symmetrical.
The HOMO of the transition state (-0.21897 a.u.) was a concern initially because it seems that there is a nodal plane right across there the C-C sigma bonds are suppose to form. Rechecking the Molecular Orbitals showed that there could be a degeneracy of orbitals involve in this instance. There is another orbital that is in very close energy to the HOMO (-0.22106). That orbital is therefore called HOMO2 in this instance. This orbital looks more likely to be involved in bonding as there's a good overlap between the orbitals of the diene and the dienophiles.
The HOMO and the LUMO of the Transition state is given as below:
| LUMO | HOMO 1 | HOMO 2 | ||
| Molecular Orbital | 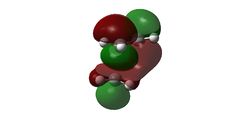
|
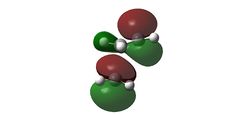
|

| |
| Energy of Transition State | Semi-empirical/AM1 | 0.02316 | -0.32499 | -0.32394 |
| HF/3-21G | 0.14241 | -0.30087 | -0.30292 | |
| B3LYP/6-G1G(d) | -0.00862 | -0.21897 | -0.22107 | |
The Intrinsic Reaction Coordinate of the Cycloaddition Transition State
The Intrinsic Reaction Coordinate method is run with using HF/3-21G and N=50. The minimum point was found after N=44 and the IRC plot and its corresponding animation to forming the cycloaddition adduct is as given as below:
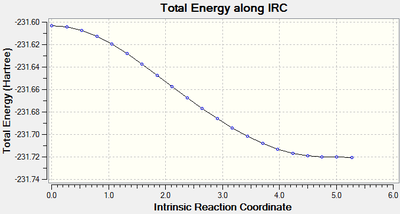
Stretches that produce the imaginary frequency
Part 3
Introduction
Transition State Structures of Diels Alder Reaction
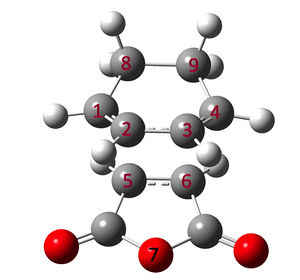
The Transition states of the Diels Alder Reaction between Maleic Anhydride and Cycloehexa-1,3-diene is shown below. The maleic acid can react with the Cycloehexa-1,3-diene with the anhydride functional group pointing towards the diene (endo) or outwards (exo). Both approaches lead to different transition states.
| Endo Transition State | Exo Transition State | ||||||
|
|
Energy of Transition State Structures of Diels Alder Reaction
The exo transition state and the endo transition state are found by using the Normal and QST2 method. Different level of theories are used in this instance. Their energies are given as below.
| Bond Lengths of the Transition States | |||
| Semi-empirical/AM1 | HF/3-21G | B3LYP/6-31G(d) | |
| Exo Transition State | -0.05041984 | -605.60359125 | -612.67931093 |
| Endo Transition State | -0.05150477 | -605.61036822 | -612.68339678 |
Bond Length of Transition State Structures of Diels Alder Reaction
The through space distance between the C and O is a subject of interest. In the exo product, there will be steric repulsion between O7 and C8/C9 while in the endo product, there will be clashes betweem O7 with C2/C3. From the table, it is obvious that the exo product is less sterically hindered as the distance between the C2 and C3 to the Oxygen atom is further than the endo product. Hence, it is expected that the exo product would be the thermally favourable product. However, in reality, the formation of endo products predominates.
Although the exo product is more stable in respect to the endo product, the transition state of the endo product is more favourable due to the contribution from the secondary orbital overlap.
Imaginary Frequency of Transition State Structures of Diels Alder Reaction
| Endo Transition State Imaginary Frequency | Exo Transition State Imaginary Frequency |
| -447.05 | -448.48 |
|
|
Molecular Orbitals of Transition State Structures of Diels Alder Reaction
HOMO and LUMO of Endo Transition State
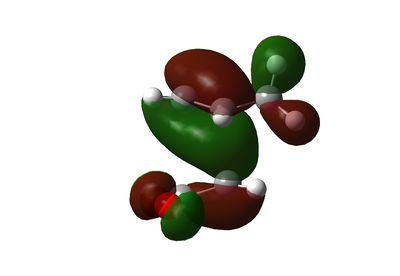
As can be seen on the HOMO of both the exo and the endo transition state, there's a flow of electron froms the diene (two sp2 carbon) to the dienophile (one sp2 carbon) forming the green bonding MO.
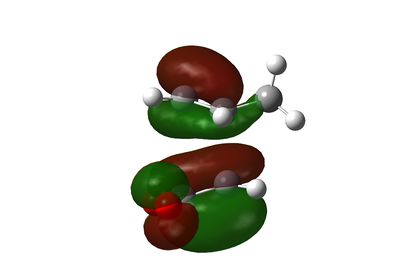
HOMO and LUMO of Exo Transition State
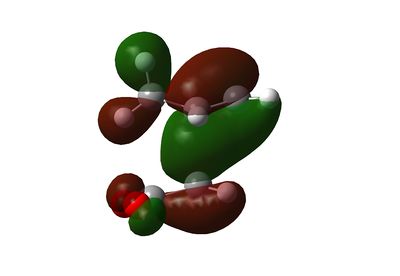

The endo transition state is more energetically favourable due to a secondary orbital effect interaction as can be seen on the HOMO-1 of the endo transition state. Such interaction is absent on the exo transition state.
Secondary Orbital Effect
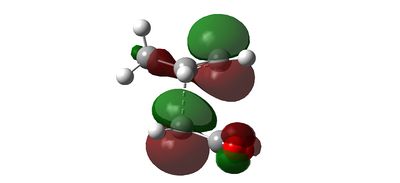
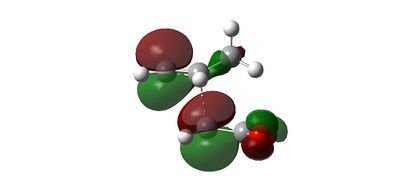
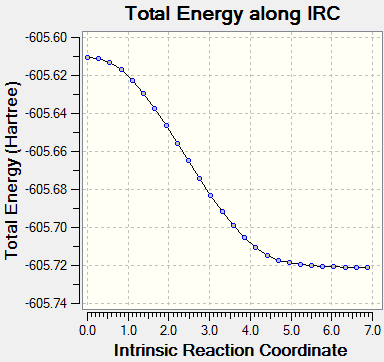
Intrinsic Reaction Coordinate of Transition State Structures of Diels Alder Reaction
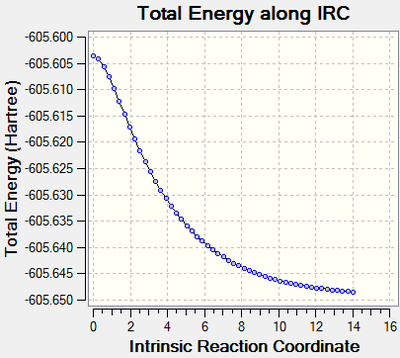
The endo transition state would lead to the endo product while the exo transition state would lead to the exo product. To confirm this, IRC method is employed using HF/3-21G method.
The results are as below:
| Endo Transition State IRC | Exo Transition State IRC |
| -447.05 | -448.48 |
|
|
|
|
As can be seen from the graph, the exo product is more energetically favourable to form than the endo, but the transition state of the endo is of a lower energy than the exo, making it the kinetic product in the Diels Alder reaction.
In all these calculations, the Gibbs Free Energy is neglected because the endo and the exo both has the same bond breakage and bond formation.
Bibliography
1. J. Phys. Chem. A, 2003, 107 (8), pp 1175–1180
2. J. Am. Chem. Soc., 1995, 117 (6), pp 1783–1788
3. Acc. Chem. Res., 1996, 29 (2), pp 67–75
4. Mol. Phys., 2001, 100(4), pp 441-446