Rep:Mod:MaxSLab
Second Year Comp Lab
EX3
BH3
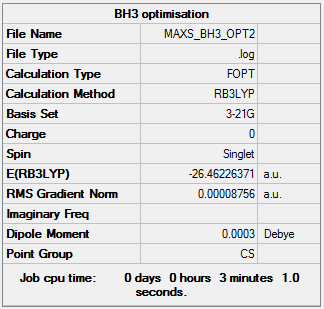
Pre-optimisation - B3LYP/3-21G
Pre-optimisation Summary Page
BH3 Pre-optimisation Item Section
Item Value Threshold Converged?
Maximum Force 0.000217 0.000450 YES
RMS Force 0.000105 0.000300 YES
Maximum Displacement 0.000919 0.001800 YES
RMS Displacement 0.000441 0.001200 YES
Predicted change in Energy=-1.635268D-07
Optimization completed.
-- Stationary point found.
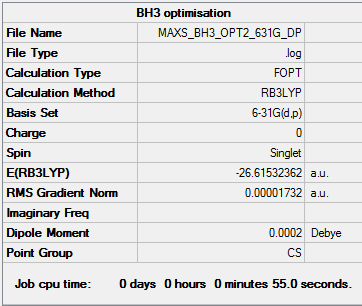
Optimisation - B3LYP/6-31G(d,p)
BH3 Optimisation [basis set = 6-31G(d,p)] Item Section
Item Value Threshold Converged?
Maximum Force 0.000018 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000089 0.001800 YES
RMS Displacement 0.000048 0.001200 YES
Predicted change in Energy=-2.973150D-09
Optimization completed.
-- Stationary point found.
Frequency .log file
MAXS BH3 FREQ.LOG B3LYP/6-31G(d,p)
Low Frequency lines for BH3 Frequency Analysis
Low frequencies --- -7.2875 -7.2549 -0.0289 -0.0004 0.6882 6.3985 Low frequencies --- 1163.0014 121 3.1570 1213.1572
Optimised BH3 Molecule |
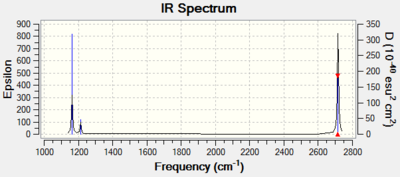
BH3 Vibrational Spectrum
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2’’ | yes | out-of-plane bend |
| 1213 | 14 | E’ | very slight | in-plane bend |
| 1213 | 14 | E’ | very slight | in-plane bend |
| 2582 | 0 | A1’ | no | symmetric stretch |
| 2715 | 126 | E’ | yes | asymmetric stretch |
| 2715 | 126 | E’ | yes | asymmetric stretch |
BH3 MO Diagram

The predicted LCAOs look very similar to the 'real' MOs. In terms of predicting phases and nodes both the representations agree. This gives credit to qualitative MO diagrams as a useful and accurate method of presenting MOs.
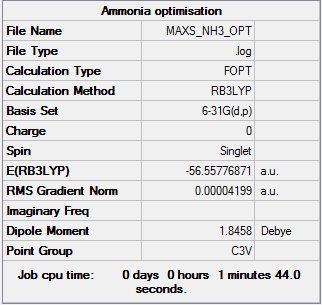
NH3
Optimisation - B3LYP/6-31G(d,p)
NH3 Optimisation [basis set = 6-31G(d,p)] Item Section
Item Value Threshold Converged?
Maximum Force 0.000060 0.000450 YES
RMS Force 0.000040 0.000300 YES
Maximum Displacement 0.000369 0.001800 YES
RMS Displacement 0.000162 0.001200 YES
Predicted change in Energy=-2.259208D-08
Optimization completed.
-- Stationary point found.
Frequency .log file
MAXS NH3 FREQ.LOG B3LYP/6-31G(d,p)
Low Frequency lines for NH3 Frequency Analysis
Low frequencies --- -30.2465 -30.2464 -27.9012 0.0011 0.0020 0.0040 Low frequencies --- 1088.3845 1693.7755 1693.7755
Optimised NH3 Molecule |
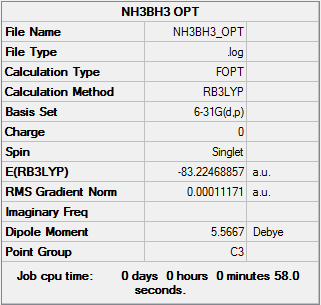
NH3BH3
Optimisation - B3LYP/6-31G(d,p)
NH3BH3 Optimisation [basis set = 6-31G(d,p)] Item Section
Item Value Threshold Converged?
Maximum Force 0.000233 0.000450 YES
RMS Force 0.000083 0.000300 YES
Maximum Displacement 0.000981 0.001800 YES
RMS Displacement 0.000370 0.001200 YES
Predicted change in Energy=-4.050297D-07
Optimization completed.
-- Stationary point found.
Frequency .log file
MAXS NH3BH3 FREQ.LOG B3LYP/6-31G(d,p)
Low Frequency lines for NH3BH3 Frequency Analysis
Low frequencies --- -0.0261 -0.0083 -0.0026 9.6692 9.6774 37.9658 Low frequencies --- 265.3241 634.4274 639.1700
Optimised NH3BH3 Molecule |
Dissociation Energy
E(NH3)= -26.61532 a.u.
E(BH3)= -56.55779 a.u.
E(NH3BH3)= -83.22469 a.u.
ΔE=[E(NH3)+E(BH3)]-E(NH3BH3)
ΔE= 0.05158 a.u.
ΔE= 135.4 kJ/mol
This bond is weak, it is lower than the O-O bond (190 kJ/mol) - a bond that breaks easily.
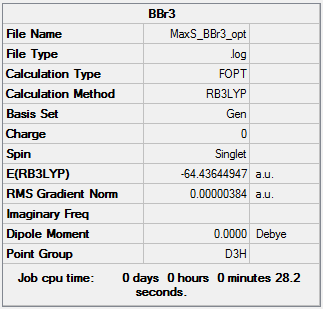
BBr3
Optimisation - B3LYP/6-31G(d,p)
NH3 Optimisation [basis set = 6-31G(d,p)] Item Section
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.085990D-10
Optimization completed.
-- Stationary point found.
Frequency .log file
MaxS BBr3 freq.log B3LYP/6-31G(d,p)
Low Frequency lines for BBr3 Frequency Analysis
Low frequencies --- -2.3055 -0.0029 -0.0018 0.0774 0.7534 0.7534 Low frequencies --- 155.9402 155.9405 267.6894
Optimised BBr3 Molecule |
Project Section
Optimisation and Freq analysis
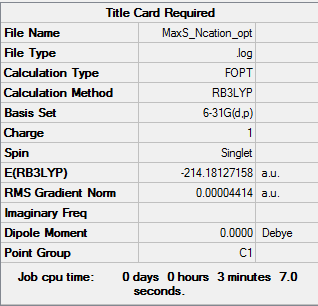
[N(CH3)4]+
Optimisation of [N(CH3)4]+ B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000071 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000421 0.001800 YES
RMS Displacement 0.000124 0.001200 YES
Predicted change in Energy=-9.303723D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -13.3480 -0.0008 -0.0008 -0.0007 5.7088 8.9200 Low frequencies --- 183.8793 289.3873 289.6979
[N(CH3)4]+ |
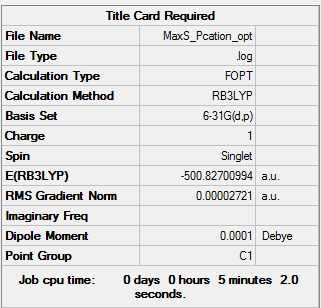
[P(CH3)4]+
Optimisation of [P(CH3)4]+ B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000144 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000664 0.001800 YES
RMS Displacement 0.000264 0.001200 YES
Predicted change in Energy=-1.699833D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -4.8625 -0.0025 -0.0020 -0.0017 5.8742 13.5041 Low frequencies --- 156.7646 191.9007 193.0045
[P(CH3)4]+ |
Charge Distribution Differences
| [N(CH3)4]+ | [P(CH3)4]+ |
|---|---|
 |
|
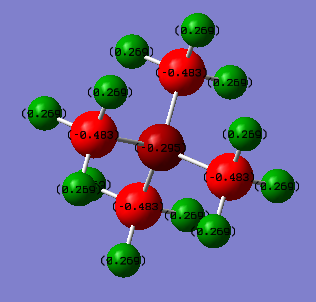
For [N(CH3)4]+ we see that the positive charge is dispersed entirely on the hydrogen atoms, while the negative charge sits primarily on the carbons, with a bit of it sitting on the central nitrogens
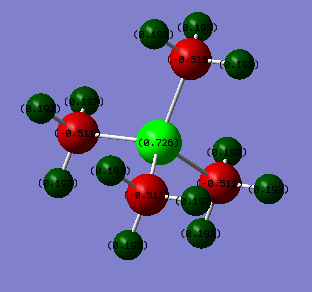
For [P(CH3)4]+ the central phosphorus atom holds a large amount of positive charge, with the hydrogens holding some postive charge as well - though less than seen on the hydrogens in [N(CH3)4]+.
The main difference between the two is the charge on the central atom, [N(CH3)4]+ has its central N atom weakly negative and [P(CH3)4]+ has the central P atom strongly positive.
Traditional description of [NR4]+
The traditional description of [NR4]+ places the formal positive charge on the central nitrogen. This suggests the nitrogen is positively charged, it has given up a lone pair to become tetravalent and is electron deficient.
Through the charge distribution that was calculated for [N(CH3)4]+, we can see that nitrogen does not hold the positive charge, rather the the hydrogens do. The nitrogen hold a negative charge.
Ng611 (talk) 22:04, 15 May 2018 (BST) Good analysis. I'd add a comment about the summation of the partial charges to +1 and another comment on how the symmetry affects the positive charge of the molecule.
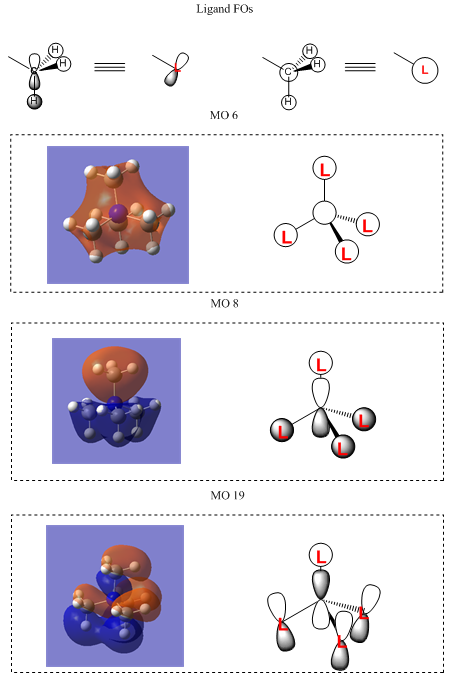
MOs of [N(CH3)4]+ with LCAO representations
Ng611 (talk) 22:05, 15 May 2018 (BST) Good LCAO analysis. It would have been useful to include some more complex orbitals, incorporating different frontier orbitals.
Ng611 (talk) 22:11, 15 May 2018 (BST) Good report. Your IR analysis was correct but you needed to discuss why only three bands were observed in the IR spectrum for 6x vibrational modes. Your charge analysis for PMe4 was also a little off, although your results were approximately accurate and your rationalisation was good. MO analysis for PMe4/NMe4 was good although would have preferred you analyse some of the more complex MOs.