Rep:Mod:MaxBridge
Introduction
A potential energy surface (PES) is used to show the energy of a system as a function pof the positions of the atoms in that system. A PES can be used to determine whether a transition state occurs during a reaction, in which a species is made that is different to the reactants and the products. When evaluating the stationary points on a PES, ie when the gradient is zero, it is found that the minima points are where the reactants and products are located and the maxima occurs when a transition state occurs. To confirm where the minima and maxima are the second derivative is taken and are found to be postive and negative respectively. Furthermore through using gaussian it can be shown that a transition sate will a negative frequency and the reactants and products will not.
During this report there will be three different exercises explored using Gaussian in which the reactants, products and transition states were all optimised at the PM6 level along with frequency calculations also performed. For exercise 2 the products were also ran at the B3LYP/6-31(d) level. An IRC calculation was ran for every reaction in order to show the reaction coordinate.
Nf710 (talk) 21:38, 16 April 2017 (BST) Remember the PES has the dimensionality of the number of degrees of freedom 3N-6. at the TS only one of these has negative curvature. This is the reaction coordinate.
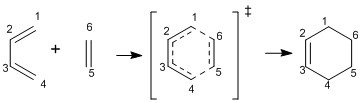
Exercise 1: Reaction of Butadiene with Ethylene
This reaction has been analyzed by optimizing the reactants and transition state using Gaussian at the PM6 level. The presence of a transition state was confirmed by running a frequency calculation at the PM6 level of the transition state and a negative frequency of -948.80 being shown.
Molecular Orbital Analysis
(Fv611 (talk) 16:24, 5 April 2017 (BST) You used the wrong symmetry labels (u/g rather than a/s). Also, you seem to have assigned lower energy to TS MOs resulting from antibonding interactions of the reactants FOs, whereas the bonding interactions should have the lowest energies. Also, the drawing of your LUMO+1 orbital is wrong.)
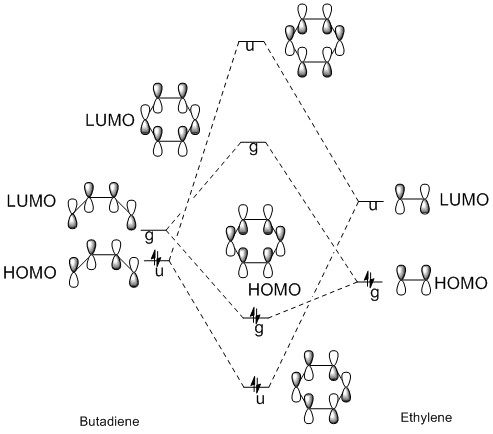
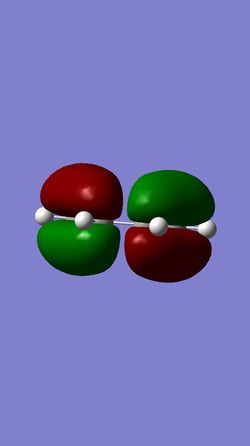
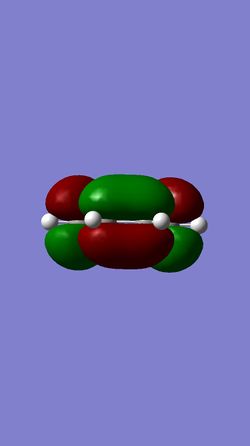
The molecular orbital diagram shows the HOMO and LUMO of both reactants and the transition state.
| HOMO of Butadiene | LUMO of Butadiene | HOMO of Ethylene | LUMO of Ethylene |
|
|

|

|
Media:ETHENEOPTMAXB.LOG
Media:BUTADIENEOPTMAXB.LOG
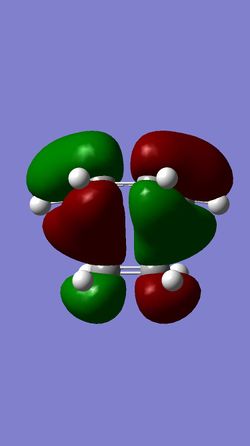
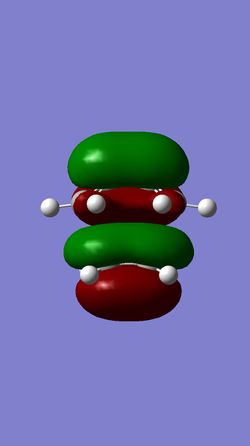
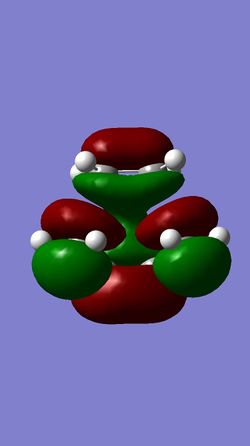
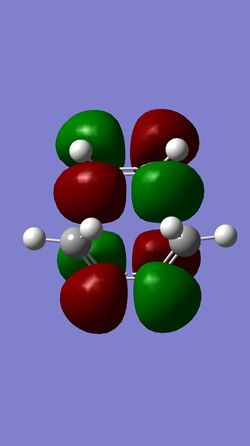
| MO 16 of TS | HOMO of TS | LUMO of TS | MO 19 of TS |
|
|
|
|
(Fv611 (talk) You have the correct MOs, but don't assign a symmetry label to any of them. Also you don't give any way of correlating your TS MOs to your MO diagram.)
The HOMO of the ethylene and the LUMO of the butadiene are both of gerade symmetry so they interact to produce molecular orbitals shown above. The HOMO of the butadiene and the LUMO of the ethylene are both of ungerade symmetry so they interact to give molecular orbital shown above. This follows a typical Diels-Alder reaction as the HOMO of butadiene is of higher energy than the HOMO of the ethylene.
(Fv611 (talk) 16:24, 5 April 2017 (BST) You do not discuss the implications of symmetry on whether a reaction is allowed or forbidden, nor talk about orbital overlaps.)
Bond Length Analysis
| Carbon Bonds | Bond Length (Å) | ||
|---|---|---|---|
| Reactants | Transition State | Product | |
| C1-C2 | 1.33528 | 1.37980 | 1.50090 |
| C2-C3 | 1.46837 | 1.41112 | 1.33784 |
| C3-C4 | 1.33528 | 1.37979 | 1.50090 |
| C4-C5 | n/a | 2.11464 | 1.54040 |
| C5-C6 | 1.32741 | 1.38177 | 1.54091 |
| C6-C1 | n/a | 2.11482 | 1.54040 |
There is an increase in bond length between C1-C2, C3-C4 and C5-C6 going from reactants to products. This is due to C1, C4, C5 and C6 all going from being sp2 hybridized to sp3 and as a result changing C1-C2, C3-C4 and C5-C6 from double bonds to single bonds. C2 and C3 remain sp2 but the bond between goes from single to double, hence the shortening in length.
The literature value for between an sp3 carbon and an sp2 carbon in cyclohexene is 1.506 Å [1] , between two sp3 carbons it is 1.541 and between two sp2 carbons it is 1.326. these are very similar values to the ones given above and show the accuracy of Gaussian.
The van der vaals radius of carbon is 1.7 Å [2] according to Pauling (1939). The distance between C4-C5 and C6-C1 is 2.11482 which is much less than double 1.7 meaning the interactions between them are much stronger than VDVs forces.
Transition State

(Dead link. Actual link is Media:BUTAANDETHANETS.LOG Tam10 (talk) 11:38, 5 April 2017 (BST))
The above vibration shows that the new C-C bonds form synchronously as the C-C bond of the butadiene is compressed whilst the terminal carbon atoms of each reactant move closer together simultaneously.
Reaction Path
Exercise 2ː Reaction of Cyclohexadiene and 1,3-Dioxole
The reactants, transition states and the exo- and endo- adducts of the product were optimized to the B3LYP/6-31G(d) level on Gaussian.
(You need to include an MO diagram for this exercise Tam10 (talk) 11:38, 5 April 2017 (BST))
Molecular Orbital Analysis
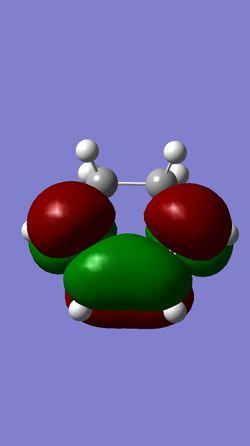
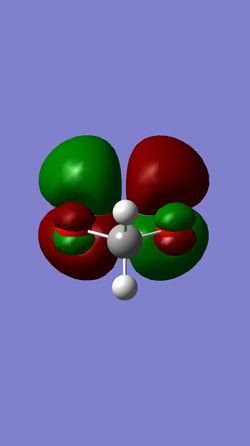
| HOMO of Cyclohexadiene | LUMO of Cyclohexadiene | HOMO of 1,3-Dioxole | LUMO of 1,3-Dioxole |

|
|

|
|
Media:CYCLOOXYGEN2.LOG Media:CYCLOHEXADIENEDFTMAXB.LOG
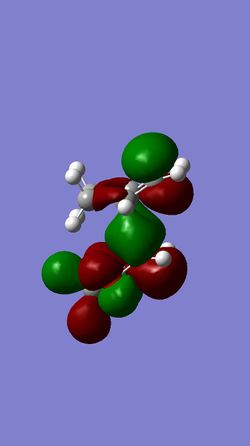
| HOMO of EXO- | LUMO of EXO- | HOMO of ENDO- | LUMO of ENDO- |
|
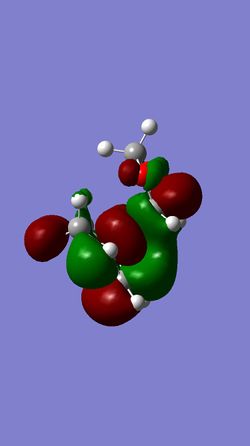
|

|
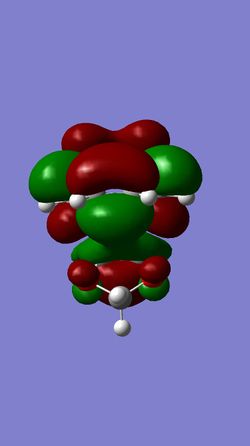
|
Media:REACTANTS TS B3LPY.LOG Media:REACTANTS ENDO TS B3LPY.LOG
This reaction is between an electron rich dienophile (Cyclohexadiene) and an electron-poor diene (1,3-Dioxole) meaning that an inverse electron demand Diels-Alder reaction occurs. The main difference between the molecular orbitals in an inverse and a standard Diels-Alder reaction is that in an inverse reaction the HOMO of the diene is lower in energy than the LUMO of the Dienophile.
Thermochemistry
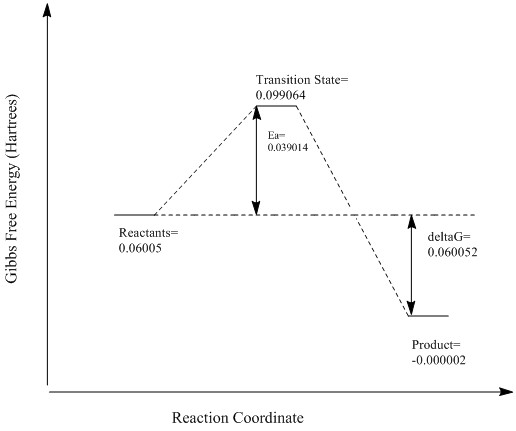
| Reactants | Transition State | Product | Ea | ΔGr | |
|---|---|---|---|---|---|
| Exo | -500.389165 | -500.329168 | -500.417323 | 0.060 | 0.028 |
| Endo | -500.389165 | -500.332111 | -500.418691 | 0.057 | 0.030 |
The endo product is shown here to have both a lower activation energy and a large change in gibbs energy meaning that it is both the kinetic and thermodynamic product. This is because in the endo product the p-orbitals on the oxygen of the 1,3-dioxole are interacting with the pi orbitals of the new carbon-carbon double bond resulting in a stabilised transition state. Furthermore there is steric hinderance in the exo transition state between the two oxygen atoms and ethane bridge.
Nf710 (talk) 22:31, 16 April 2017 (BST) You should have converted these energies to KJ mol-1 However it looks like your TS and products are correct but your reactants are slightly out. You could have backed up your arguments with diagrams
Bold text==Exercise 3: Diels-Alder vs Cheletropic==
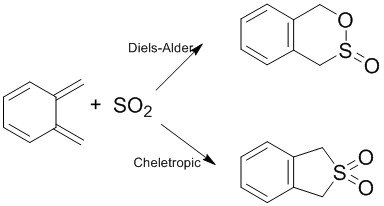
Diels-Alder: EXO
Reaction Coordinate:
IRC Path
File:Total Energy along IRC EXO.pdf Media:REACTANTSEXOIRC.LOG
Energy Profile for Exo Product
(These energy profiles should be on the same graph. That way a reader can compare the reactions more easily. We also asked for energies in kJ/mol and to normalise to the reactants. The energies here are swapped with endo Tam10 (talk) 11:38, 5 April 2017 (BST))
Diels-Alder: Endo
IRC Path
File:Total Energy along IRC ENDO.pdf Media:REACTANTSENDOIRC.LOG
The IRC shown here is in reverse resulting in it going from products to reactants.
Energy Profile for Endo Product
The IRCs of both the endo and exo reactions show the formation of a new six membered ring. It can be seen in both reaction paths that there is a delocalistion of electrons throughout the xylylene due to it being highly unstable. The reaction paths show that the exo- product is favored kinetically and thermodynamically due to it having a lower activation energy and large change in gibbs energy.
Cheletropic reaction
IRC Path
File:Total Energy along IRC CHELO.pdf
Energy Profile for Chelotropic reaction
The chelotropic reaction shows a higher activation energy in comparison to the diels-alder reaction, this is down to a five membered ring forming which is more strained than the 6 membered ring formed in the diels-alder reaction. The chelotropic reaction has a much higher change in gibbs energy than the diels-alder reaction meaning that the chelotropic product would form under thermodynamic control and the diels-alder under kinetic control.
Conclusion
In all three exercises the reactants, products and transition states were successfully optimized under the PM6, with the endo- and exo- reaction paths also being successfully optimized to the B3LYP/6-31G(d) level. This resulted in successful anaylsis of the HOMOs and LUMOs of the reactants and transition states for reactions in the first two exercises. Furthermore it lead to thermochemical data about the reaction paths being analysed for the reaction in exercises 2 and 3 which meant that the kinetic and thermodynamic products could be subsequently confirmed.
References
[1] F. H. Allen, O. Kennard, D. G. Watson, L. Brammer and A. G. Orpen, 1987, 1–19.
[2] S. S. Batsanov, 2001, 37, 871–885.