Rep:Mod:Mate Toth 19
Part 1 - Optimisation/Frequency Analysis/Basis Sets and Pseudo Potentials
Ng611 (talk) 16:42, 13 May 2019 (BST) You had the makings of a decent report here but you let yourself down with very basic mistakes (missing log files, reporting numbers to incorrect precisions, missing questions out, etc.). Your MO analysis was well done though, good job.
Error
All results and calculations are subjected to the same standardised errors below:
Energy ≈ +/- 5 kJ/mol Distance ≈ +/- 0.001 Å Bond Angles ≈ +/- 0.1° Frequencies: systemic error of around 10%
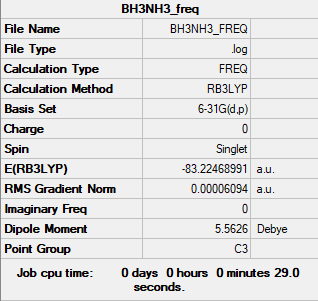
BH3
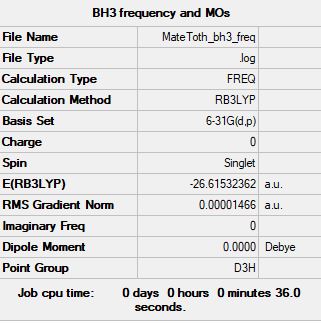
Summary Table
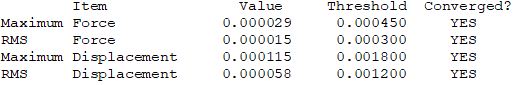
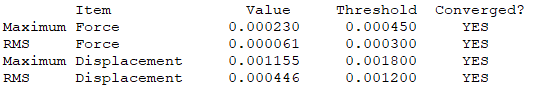
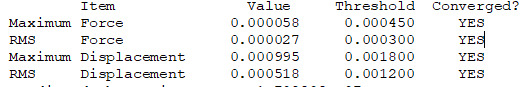
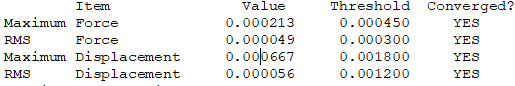
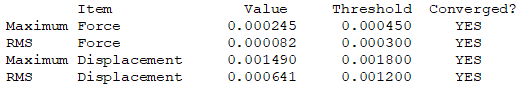
Convergence Table
Frequency analysis log file: MATETOTH_BH3_FREQ.LOG
Ng611 (talk) 16:22, 13 May 2019 (BST) You need to actually include the file to get the marks.
Low frequency table
Molecular BH3
test molecule |
Frequency Analysis
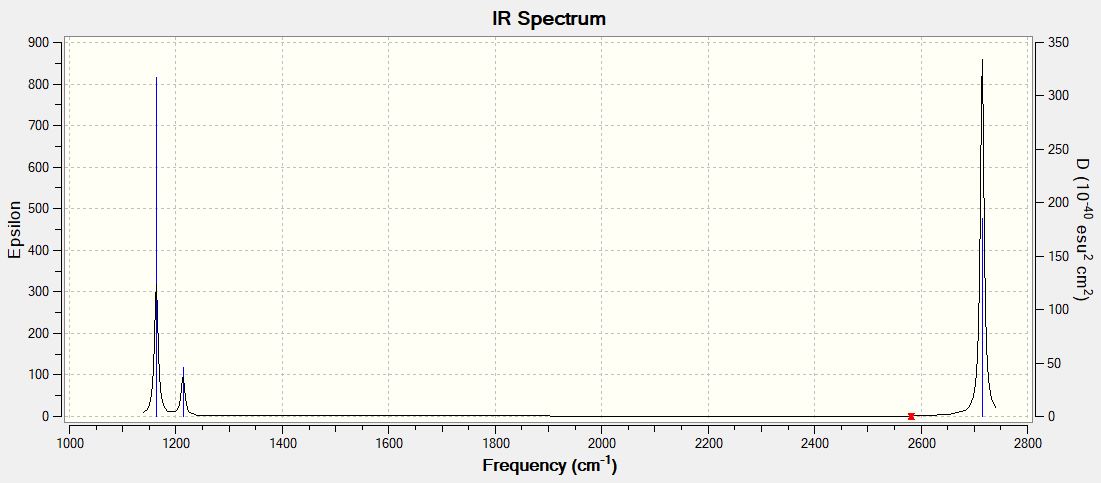
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type | |
|---|---|---|---|---|---|
| 1163 | 93 | A2 | Yes | out-of-plane bend | |
| 1213 | 14 | E | slightly | bend | |
| 1213 | 14 | E | slightly | bend | |
| 2582 | 0 | A1 | No | symmetric stretch | |
| 2715 | 126 | E | Yes | asymmetric stretch | |
| 2715 | 126 | E | Yes | asymmetric stretch |
IR spectrum of BH3, after optimisation
Ng611 (talk) 16:24, 13 May 2019 (BST) You also need to explain why 6 vibrational modes only result in 3 lines in your calculated spectrum.
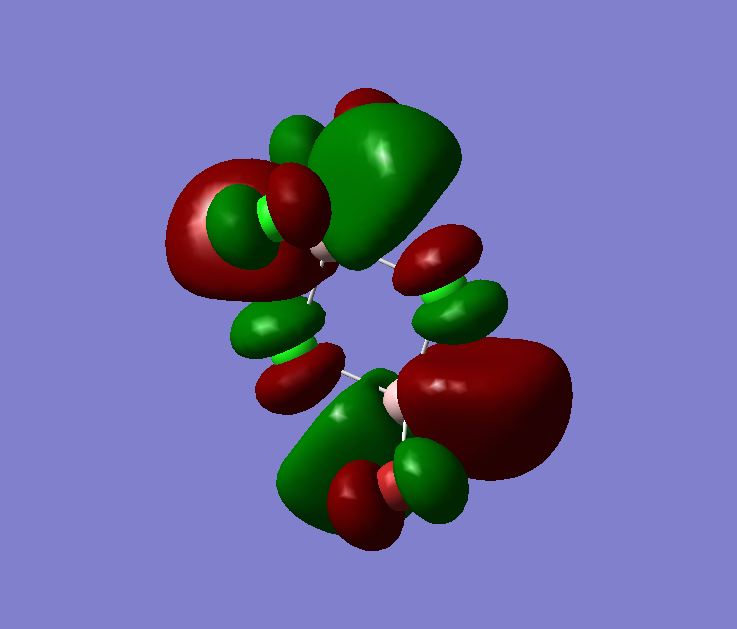
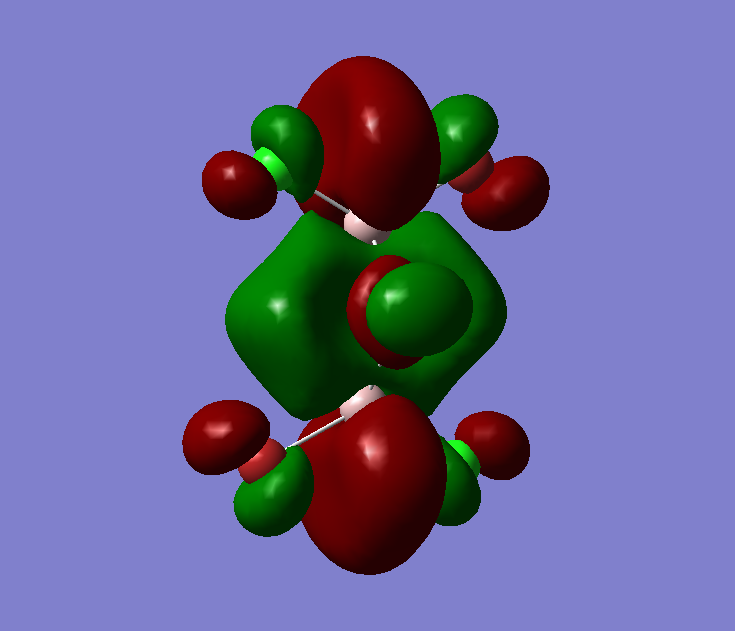
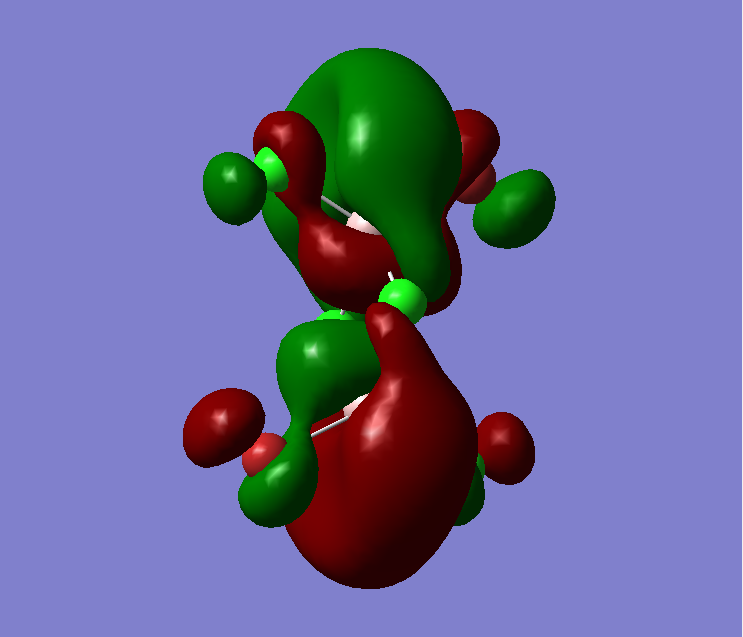
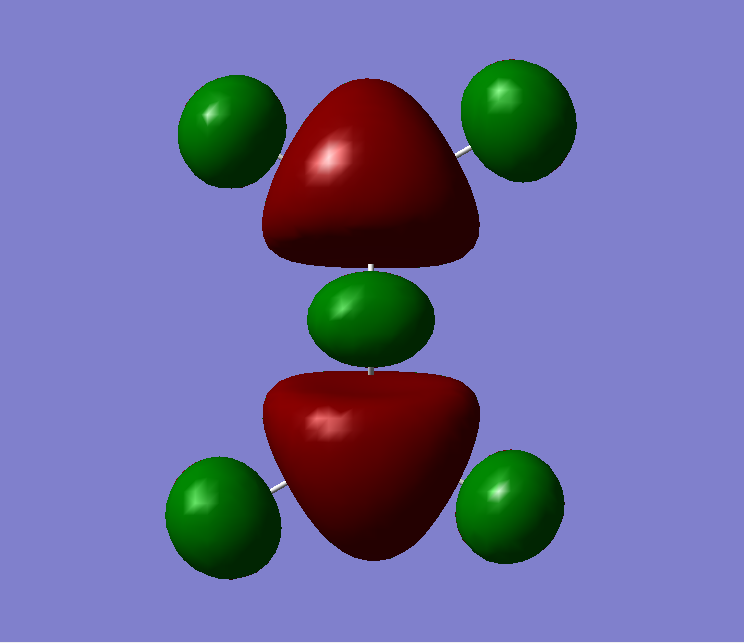
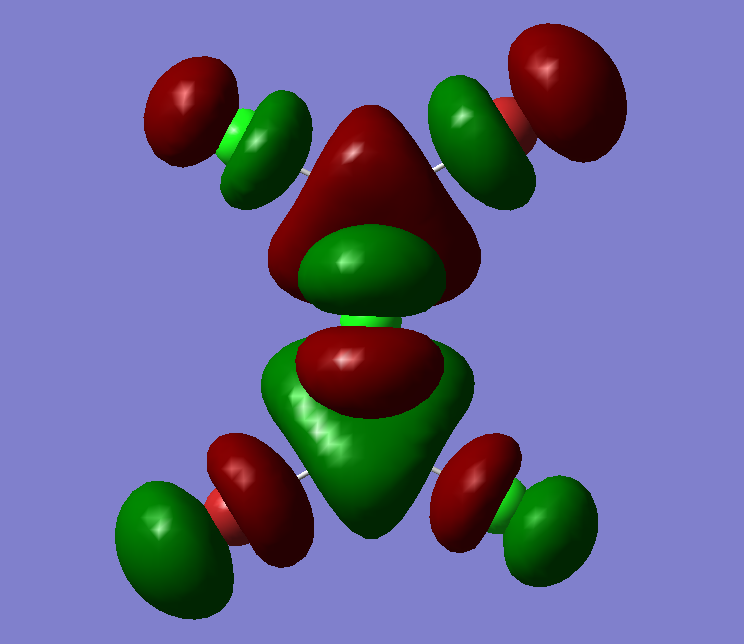
The MO diagram of BH3, both the LCAOs and real MOs are displayed
Comparison of theoretical and real MOs
The most significant difference is that the LCAOs are displayed as the cocktail of separately imagined orbitals, and therefore the final lobes are not continuous but has distinct boundaries. In comparison (see MO2 or MO8), the real MOs have continuous lobes and are not separated bu the imaginary ends to the lobes. Empiricacally, it ois much easiier to draw LCAOs without the knowledge of an expert or a software. Also, the significance of the difference in between the sizes of the fragments in the MOs due to the E proximity of fragments is smaller in real MOs.
In general, the MO theory is accurate in telling the basic stecuture of the MOs. Hence, it is very useful when we are trying to approximate the relative energy of an MO and thus the attracxtive and repulsive forces and the nuymber of nodes can be determined. However, the structure is not as accurate. This is diue to the complicated mnature of MO surfaces.
NH3
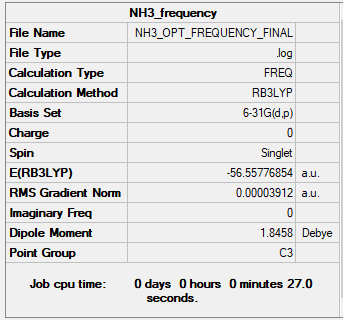
Summary Table
Ng611 (talk) 16:26, 13 May 2019 (BST) You're missing jMol images AND .log files for all of these calculations.
The point group was C3V after optimisation but changed back - repeatedly - to C3 after frequency measurements.
Convergence Table
Low Frequency Table
NH3BH3
Summary Table
The point group was C3V after optimisation but changed back - repeatedly - to C3 after frequency measurements.
Convergence Table
Low Frequency Table
Energy calculations
E(NH3)= -56.557768 au E(BH3)= -26.61532362 au E(NH3BH3)= -83.2246899 au
Difference = 0.0516 au
Difference = 135.4758 KJ/mol
Ng611 (talk) 16:29, 13 May 2019 (BST) Too many d.p. in your final reported figure. You should report values to the nearest kJ/mol
Comparison of bond strengths
The c-c single bond is 346 KJ/mol, whereas the N-n bond is 167 KJ/mol. Comparing to these values, the dative bond is a weaker than both of the single bonds in the homo nuclear diatomic molecule. But it is not weak, but medium compared to the N-N single bond.
Ng611 (talk) 16:31, 13 May 2019 (BST) Good comparisons but you need to include references for the bond enthalpies you cite.
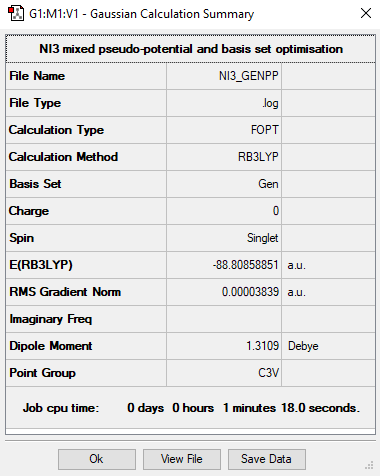
NI3
Summary Table
Ng611 (talk) 16:32, 13 May 2019 (BST) Remember to include your .log file or DSpace link. Otherwise, it's impossible to check the validity of your calculation
Convergence Table
Low Frequency Table
Molecular NI3
test molecule |
Bond length: 2.18424 A
Project-Main Group Halides
Ng611 (talk) 16:34, 13 May 2019 (BST) All of these are missing both .log files and low frequency tables.
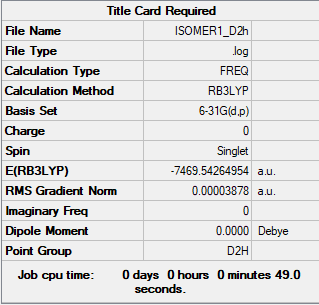
Isomer 1
test molecule |
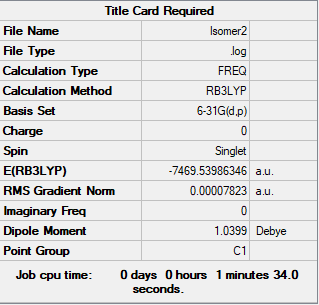
Isomer 2
test molecule |
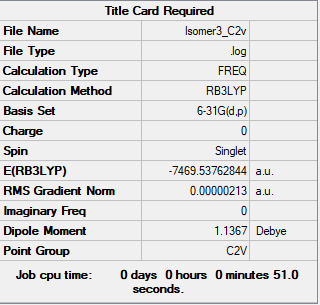
Isomer 3
test molecule |
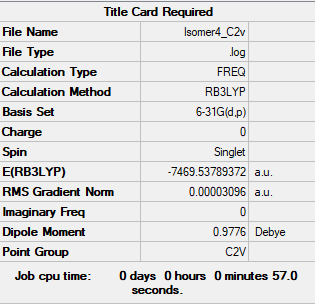
Isomer 4
test molecule |
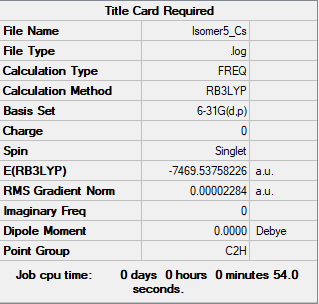
Isomer 5
test molecule |
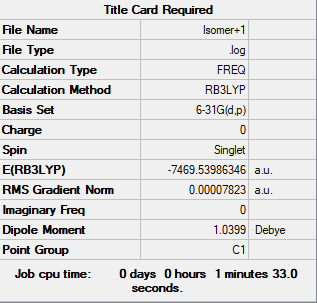
Isomer +1
After consulting with Prof. Patricia Hunt, I have decided to investigate the possibility of a sixth isomer. The relative position of Br and Cl atoms were changed but and angles were constant. In conclusion, the sixth isomer may seem to be different, but energetically is identical.
test molecule |
Energy of Isomer 1 and
The energy of Isomer 1 (bridging Br ions) and Isomer 5 (trans terminal Br ions) were calculated.
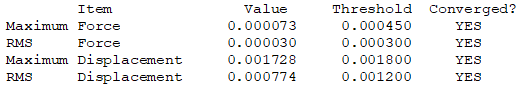
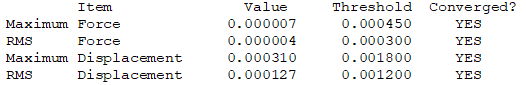
Isomer 1: -7469.54264954 au = -19611284.2246 KJ/mol Isomer 5: -7469.5375822 au = -19611270.92206 KJ/mol
E difference: 13.3 KJ/mol
Isomer 1 is more negative, which means that more E is needed to break a bond within the molecule. Therefore, according to these measurements Isomer 1 is more stable. However, theoretically, Isomer 5 should be more stable. The Cl bridge is moire resistant to dissociation as the Cl-Al overlap is better. This is due to the fact that both of these elements are in the 3rd raw of the periodic table. Whereas, Br is a 4th row element and therefore the orbital overlap with Al is less good.
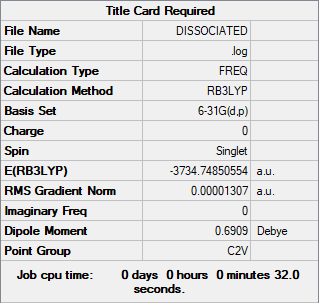
Dissociation energy.
The dissociation energy was determined by using the energy of a single molecule of 2AlCl2Br and this value was multiplied by two. The difference between the lower energy conformer from the two above (Isomer) was measure and compared to the values yielded by the single molecule.
E of 2AlCl2Br: -3734.74850554 au, giving a difference of 0.04057112 au compared to the E of Isomer 5. Thus, the overall E difference is 106.5195 KJ/mol, by which Isomer 5 is lower in E than the 2 monomers together, thus being more stable. Consequently, the dimer is the energetically more favoured configuration.,
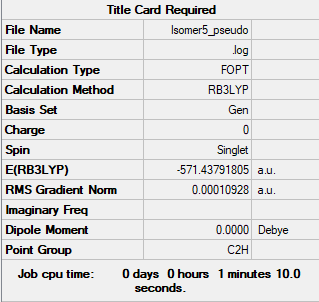
Pseudo
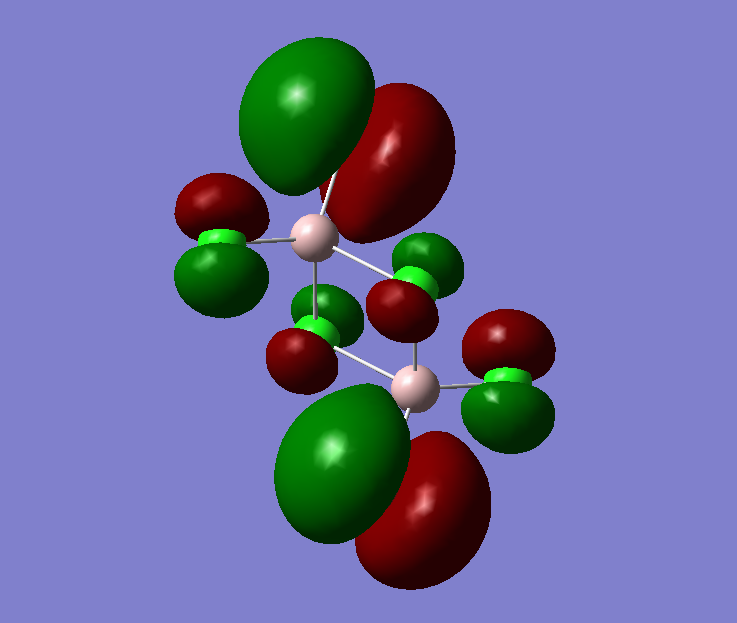
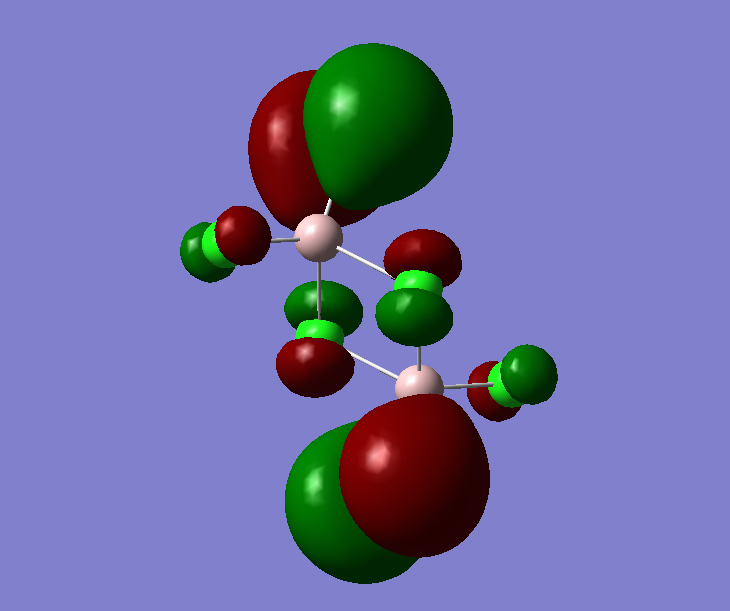
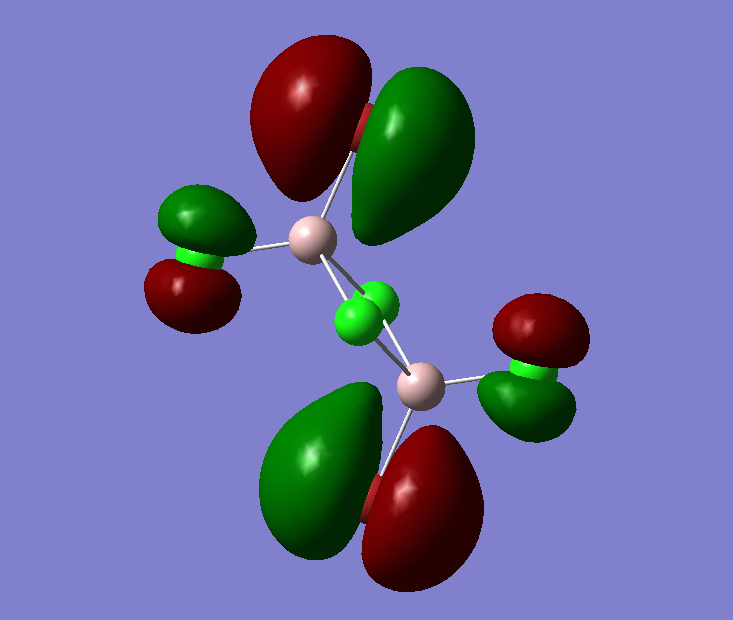
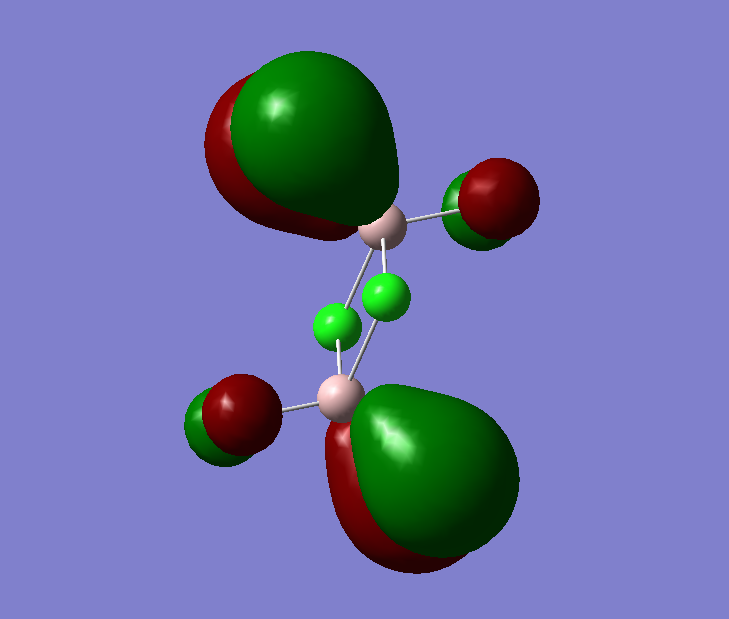
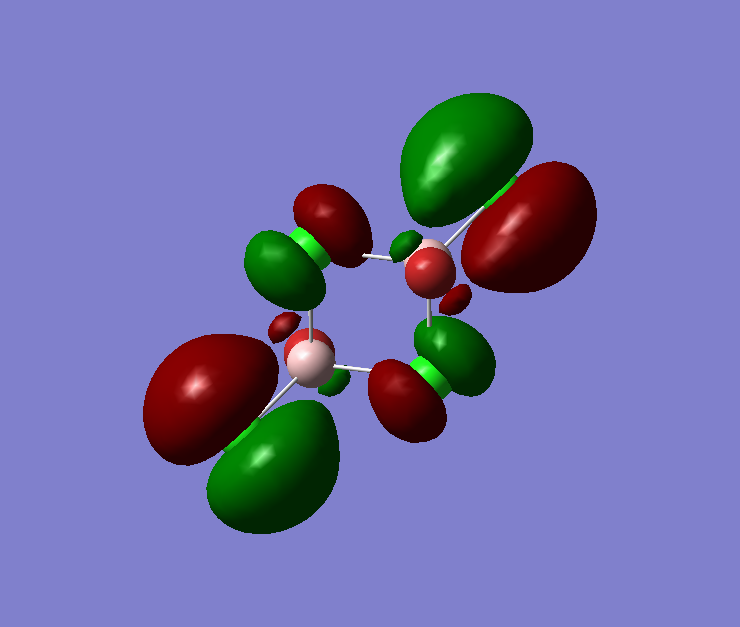
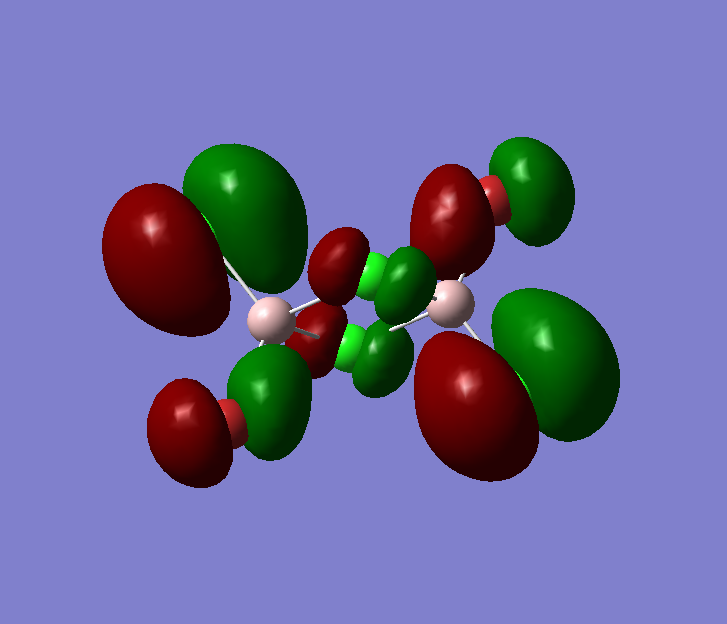
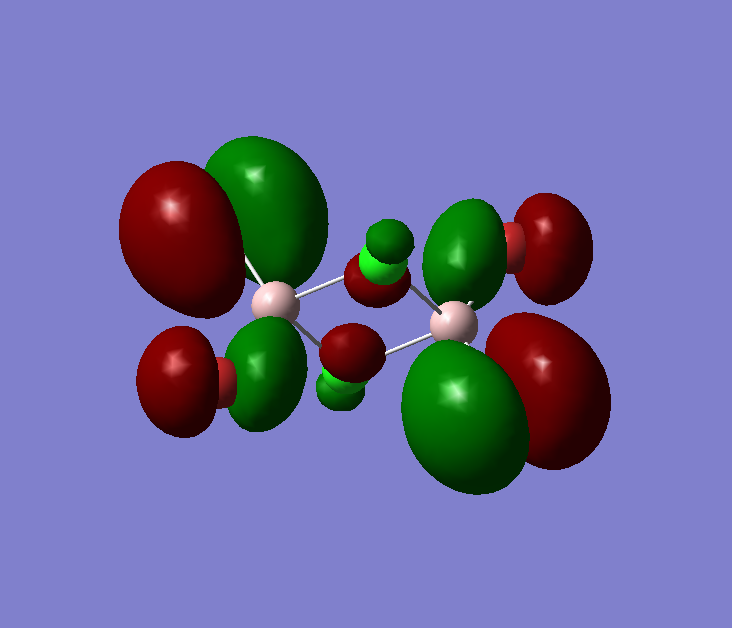
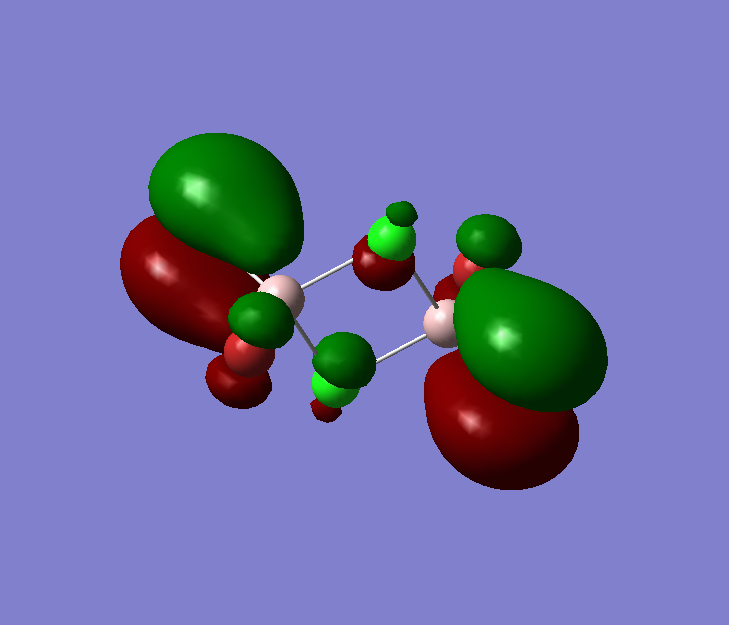
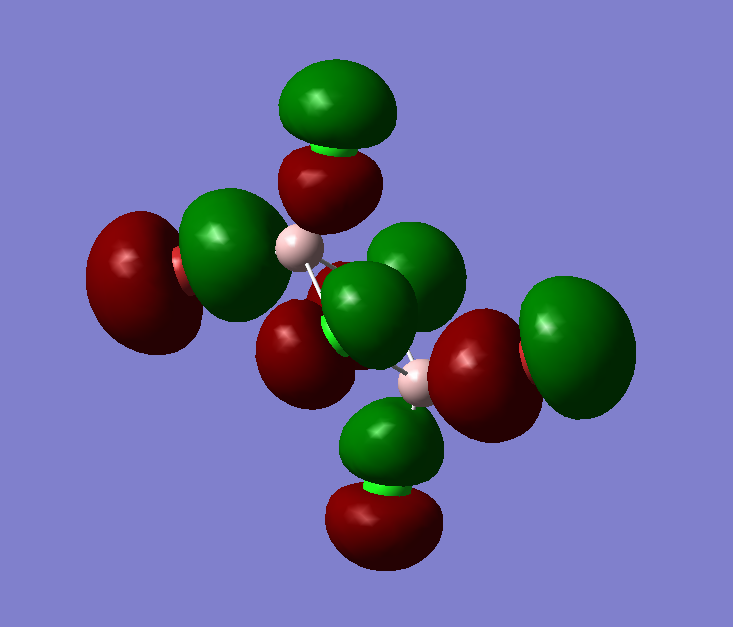
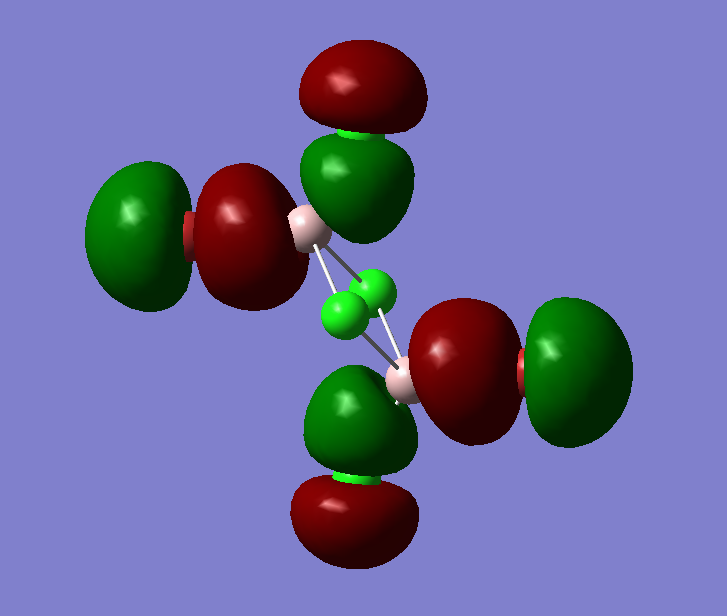
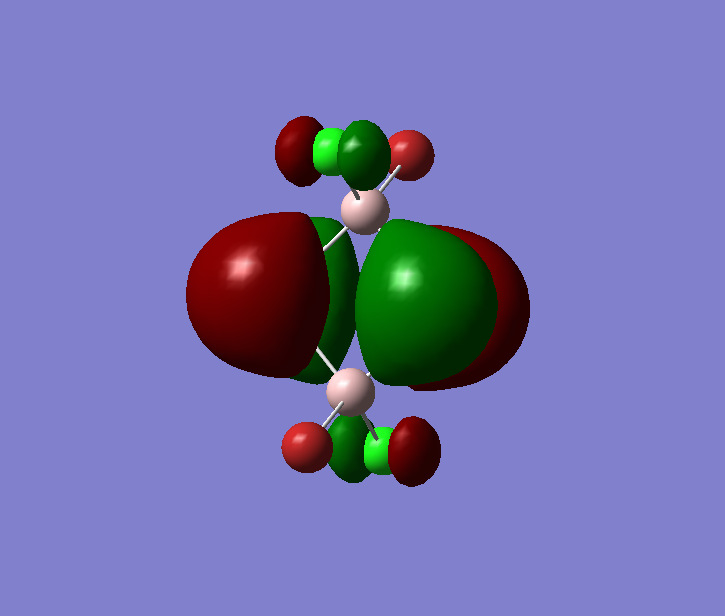
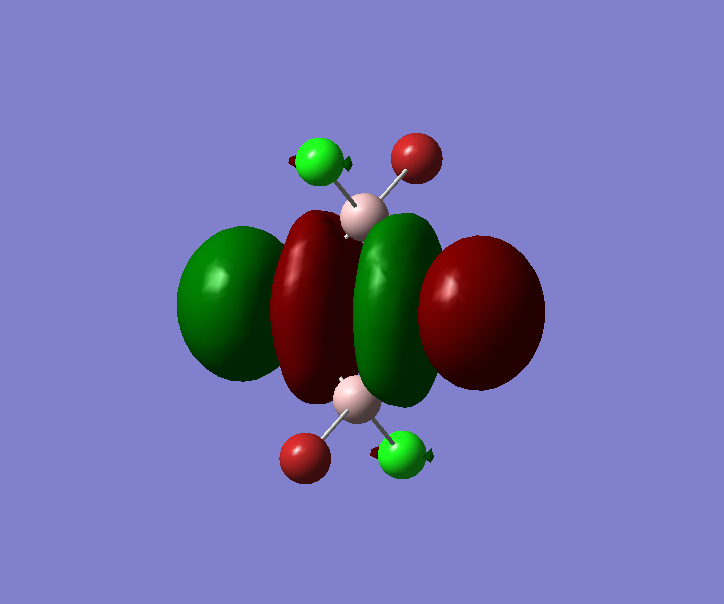
The pseudo potentials were used to get the pseudo structure of the lowest isomer from the 2, which is isomer 5. The summary of the calculation is shown below. Moreover, 5 of the unoccupied, and the valence MOs are also shown below. Al has 3, BR and Cl have 7 valence electrons, giving 48 electrons and 24 pairs on the valence MOs. In total, five unoccupied and 12 occupied MOs are shown below.
Visualise of 12 occupied and 5 unoccupied MOs
Unoccupied:
Unoccupied 5
Unoccupied 4
Unoccupied 3
Unoccupied 2
Unoccupied 1
Occupied:
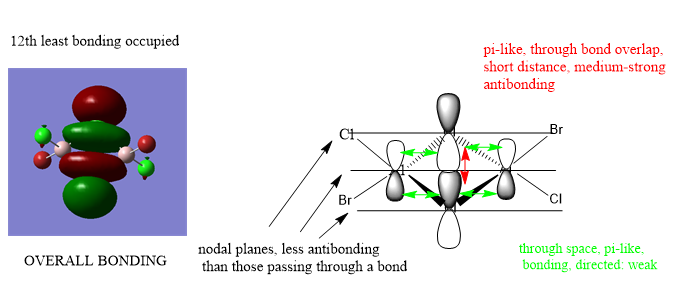
Occupied 12
Occupied 11
Occupied 10
Occupied 9
Occupied 8
Occupied 7
Occupied 6
Occupied 5
Occupied 4
Occupied 3
Occupied 2
Occupied 1
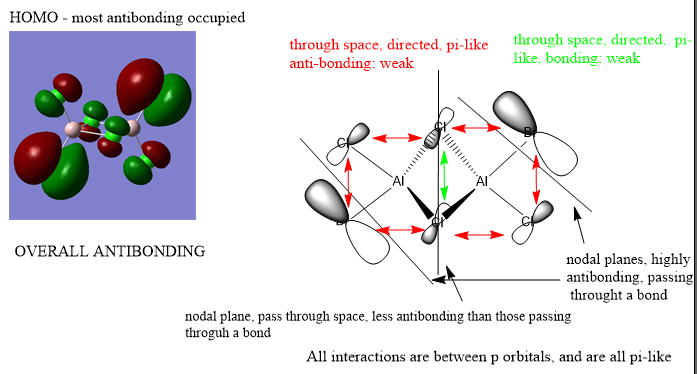
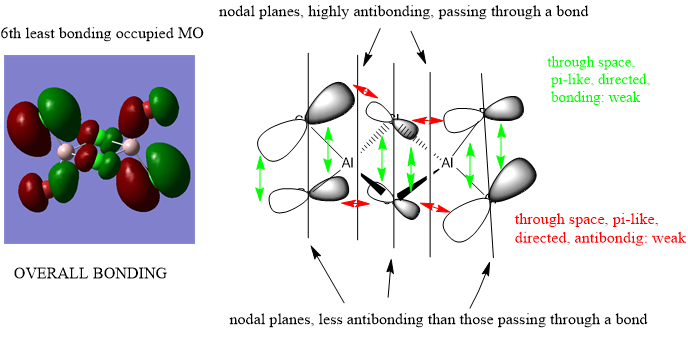
Analysis of 3 MO bonding
3 MO interactions were analysed in more detail. These are all occupied, and varied between bonding and antibonding in nature.