Rep:Mod:Mac1
NH3 Optimization
Calculation
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final Energy(a.u.): -56.55776873
RMS Gradient(a.u.): 0.00000485 Point Group: C3V
Bond Length: 1.01798
Bond Angle: 105.741
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986266D-10
Optimization completed.
-- Stationary point found.
Results
NH3 |
.log Source File: File:MC4716 NH3 OPTI 1.LOG
Vibration
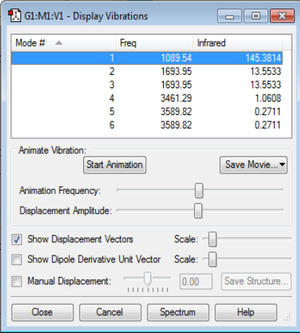
How many modes do you expect from the 3N-6 rule?
3(4) - 6 = 12 - 6 = 6
Which modes are degenerate (ie have the same energy)?
There are two degenerate mode pairs: 2-3 and 5-6.
Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Bending Vibration Modes: 1, 2, and 3
Bond Stretching Vibration Modes: 4, 5, and 6
Which mode is highly symmetric?
4
One mode is known as the "umbrella" mode, which one is this?
1
How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
3, one for each unique frequency, minus the IR inactive symmetric stretch.
Charge
Nitrogen Atom Charge: -1.125
Hydrogen Atom Charge: 0.375
The nitrogen atom, as the more electronegative atom, would have a greater percentage of the electron density and should therefore be the negatively charged atom. The hydrogen atoms should be positively charged and have a similar charge to the nitrogen, but the charge should be distributed across the all three.
N2 Optimization
Calculation
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final Energy(a.u.): -109.52412868
RMS Gradient(a.u.): 0.00000060
Point Group: D*H
Bond Length: 1.10550
Bond Angle: 180
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.400905D-13 Optimization completed. -- Stationary point found.
.log Source File: File:MC4716 N2 OPTI 1.LOG
Vibration
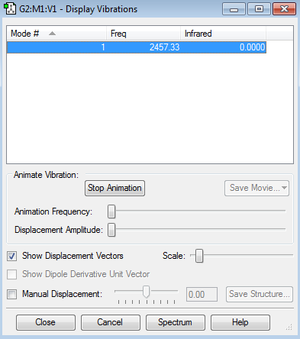
H2 Optimization
Calculation
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final Energy(a.u.): -1.17853936
RMS Gradient(a.u.): 0.00000017
Point Group: D*H
Bond Length: 0.74279
Bond Angle: 180
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
.log Source File: File:MC4716 H2 OPTI 1.LOG
Frequency
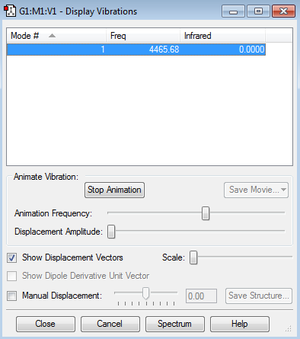
Energy of reaction
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u. = -146.48 KJ/mole
The ammonia product is more stable than the reactants as the energy of difference between the reactants and products is negative. This indicates a release of energy and product in a lower energy configuration than the products, leading to more stability in the product form.
ClF3 Optimization
Calculation
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final Energy(a.u.): -759.46531673
RMS Gradient(a.u.): 0.00006653
Point Group: CS
Bond Length: 1.72851 and 1.65166
Bond Angle: 87.150
Item Value Threshold Converged?
Maximum Force 0.000164 0.000450 YES
RMS Force 0.000067 0.000300 YES
Maximum Displacement 0.001028 0.001800 YES
RMS Displacement 0.000606 0.001200 YES
Predicted change in Energy=-1.549029D-07
Optimization completed.
-- Stationary point found.
Results
ClF3 |
.log Source File: File:MC4716 CLF3 OPTI 2.LOG
Vibration
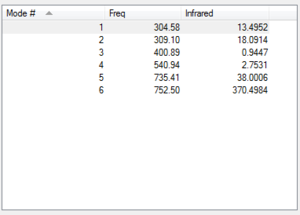
Charges
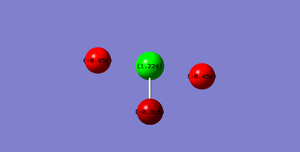
Fluorine is a much more electro-negative atom than chlorine and therefore is distinctly negatively charged. Due to the difference in bond lengths the flourines are negativly charged to different degrees. The fluorines with the longer bond length will have larger molecular orbitals. This will result in a larger orbital with more space in which electrons can reside increasing the charge density along these bonds compared to the shorter bond to the central flourine.
Molecular Orbitals
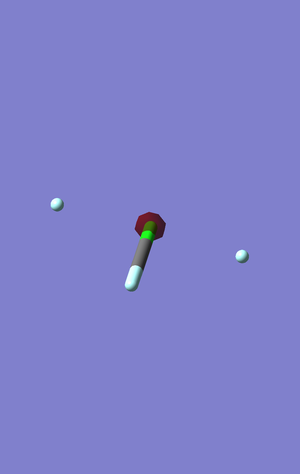
This is the lowest occupied orbital. It is the central orbital for the chlorine's s1 orbital electrons. Due to its very low energy level, -101.79316 a.u., it does not contribute to bonding or antibonding.
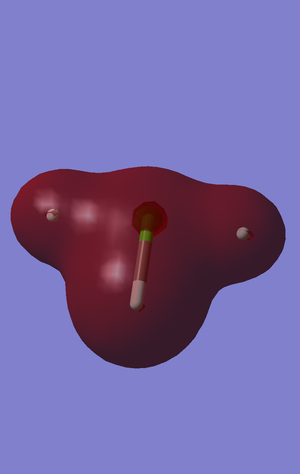
This is the first sigma bonding orbital. It has contributions from all s2 orbitals in every atom. This can be seen in its complete covering of all atoms. This orbital has a large impact on the overall strength of the bonds. This is the lowest bonding orbital, but it much higher in energy that the non-bonding pi orbitals of the chlorine below it, -1.28152 compared to -7.43166 a.u.
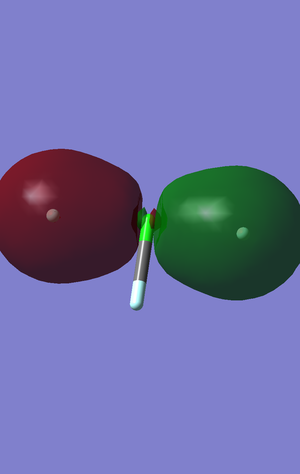
This is the first pi bonding orbital between the two outer fluorines and the central chlorine. This orbital exhibits sp hybridized character as there is a large and small node. The contributing atomic orbitals are the p orbitals that are parallel to the bond axis in the outer fluorines and the central chlorine. This is the last of the low energy bonding orbitalsorbitals and the is a large increase in energy following this orbital.
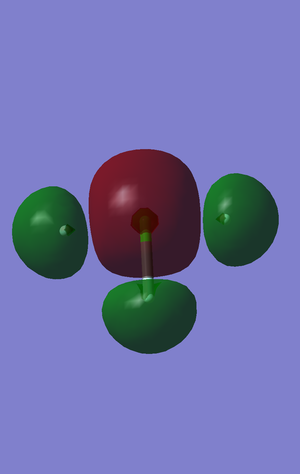
This molecular orbital is an occupied sigma antibonding orbital. This is the first antibonding orbital that is paired to an active bonding orbital(see second molecular orbital). This bonding orbital is filled by the 23-24 electrons out of 44 and of the occupied orbitals is located centrally at -0.89009 a.u.
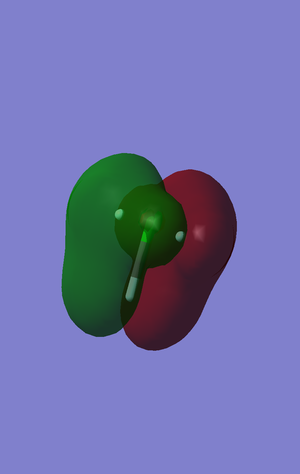
This molecular orbital shows the conjugated pi system that exists despite the lack of double bonds. The lone pairs in the p atomic orbitals that are orthogonal to the bonding axis can still interact in the molecular orbitals and form the conjugated system seen. The p atomic orbitals that make up this system are also orthogonal to the plane of the whole compound and therefore are located directly below in energy to the other pi system, which must be distorted in the plane of the compound to fully conjugate, -0.53814 and -0.47541 a.u. respectively.
ClH3 Optimization
Calculation
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final Energy(a.u.): -461.79881375
RMS Gradient(a.u.): 0.00000750
Point Group: CS
Bond Length: 1.63183 and 1.28279
Bond Angle: 85.412
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.000102 0.001800 YES
RMS Displacement 0.000058 0.001200 YES
Predicted change in Energy=-1.459464D-09
Optimization completed.
-- Stationary point found.
Results
ClH3 |
.log Source File: File:MC4716 CLF3 OPTI 1.LOG
Vibration
Charges
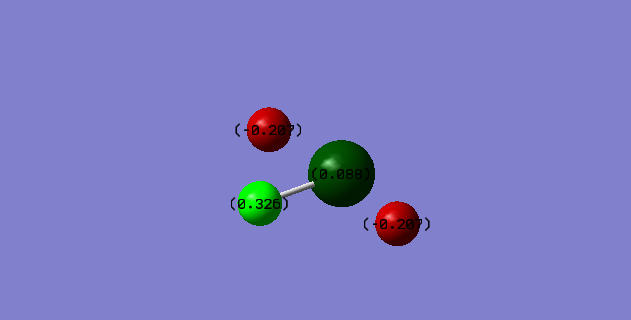
The charge distribution shows that the non-central hydrogens are the most electro-negative and are the only negatively charged atoms. The central chlorine is almost neutral with a very small positive charge of 0.088. The most positive atom, counter intuitively, is the central hydrogen atom despite its distance from the lone pairs. This can be explained by visualizing the molecular orbitals. The larger bond lengths are accompanied by larger molecular orbitals. This gives more electron density around the long bonded hydrogens and less in comparison to the shortly bonded one.
Molecular Orbitals
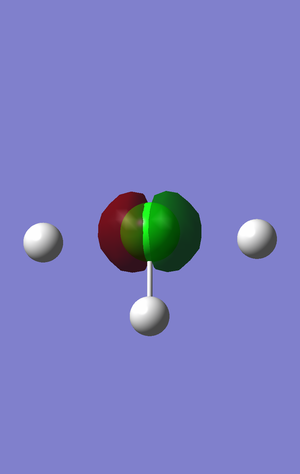
This orbital is buried so deep in the chlorine atom that it is not visible in the model. It is the core orbital of the s1 chlorine orbital and will have no effect on bonding. This sigma orbital, -101.65878 a.u., is approximately an order of magnitude lower in energy than the next lowest orbital.
This is the simple sigma molecular orbital between the fluorine s2 and hydrogen s2 atomic orbitals. This orbital contributes strongly to the bonding as it is the lowest energy bonding orbital. While it is the lowest bonding orbital its energy of -7.32595 a.u. does not compare to the lowest sigma orbital mentioned above.
Comparison of ClH3 and ClF3
Through modeling these closely related compound new insights can be drawn. First of all the stark contrast between the charge distributions shows there is significant difference in the interactions of hydrogen with chlorine and fluorine and chlorine. The hydrogen is capable of sustaining an positive and negative distribution, while the fluorine is solidly negative. This shows that the electro-negativity of chlorine and hydrogen must be more similar than chlorine and flourine as no one atom dominates.
Similarities and differences arise in the molecular orbitals. While the deepest energy levels are similar as they are located in the core of the chlorine atom and are unaffected by bonding, the similarities stop there. The fluorine orbitals are slightly higher in energy due to stronger ionic effects and more similar sized atoms in the bonding. In addition there is pi bonding involved in the system. This was not present in ClH3 due to the lack of p level electrons in the hydrogen atom.
The overall shape and bond length, while different due to the larger size of fluorine, are similar in there overall construction. The central atom is more closely bonded and the bond angle being less than 90 degrees due to the interaction with the lone pairs.
At first glance the molecules look very similar, when their deeper characters are explored stark contrasts can be found.
