Rep:Mod:MaHardst
Introduction
This wiki page includes a summary of the general properties,the vibrational analysis,the charge analysis and a Jmol image of four different molecules- NH3, N2, H2 and ClF. All of the information included was calculated using the "Gaussian" software package, the results and visual depictions were hence viewed in the corresponding "Gaussview" software. Using the optimised bond energies produced from this computational analysis of NH3, N2 and H2, the enthalpy change of reaction for the Haber-Bosch process (N2 + 3H2 -> 2NH3) was calculated and compared to a literature values to determine the percentage error in the reaction enthalpy. Furthermore, the molecular orbitals of ClF were imaged, and through visual analysis, the character of each MO could be described, in terms of its constituent orbitals and its bonding nature.
NH3 Molecule
General Information
A summary of general information obtained from the results of the optimisation of the NH3 molecule in the "Gaussian" software package is included below.
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy, E(RB3LYP): -56.55776873au
RMS Gradient Norm: 0.00000485au
Point Group: C3V
Optimised NH Bond Distance: 1.01798Å
Literature NH Bond Distance in NH3: 1.012Å[1] Thus, the computationally modelled bond length has a 0.59% percentage uncertainty, which is a very high accuracy, indicating that the "Gaussian" optimisation of NH3 produces an excellent approximation to the actual physical molecule, and therefore, this optimisation could be relied upon to use in further analysis, i.e. in calculating other values of properties associated to the NH3 molecule.
Optimised H-N-H Bond Angle:105.741°
Item Table
The table below is found within the 'real' results text file produced from the optimisation of the NH3 in the "Gaussian" software package. It is included in this report as proof that this optimisation reached completion.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS Force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000072 | 0.001800 | YES |
| RMS Displacement | 0.000035 | 0.001200 | YES |
Predicted change in Energy=-5.986282D-10 Optimization completed. Stationary point found.
JMol Image
Included below is a Jmol rotatable image of the optimised NH3 molecule, generated in the "Gaussian" software.
NH3 Molecule |
The optimisation file is linked here
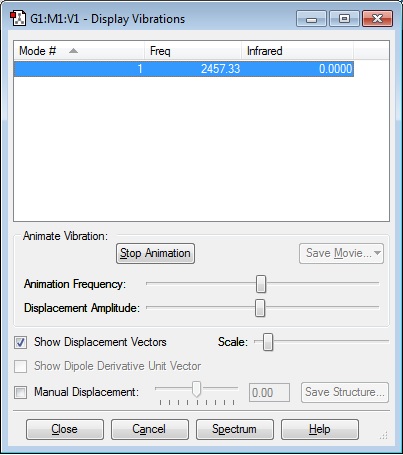
Vibrational Analysis
Using the optimised NH3 molecule, an analysis of the vibrational modes was performed in the "Gaussian" software, to determine the frequency at which distinct bond stretches/bends are produced, and the corresponding value (i.e. intensity of absorption of IR radiation) they would produce in a IR spectrum of the molecule. Included below are all of the different IR absorptions that would be shown due to the different vibrational modes available to the molecule.
As is evident, there are no negative frequencies given in the above table, indicating that the optimisation has been successful.
Number Of Modes Expected:6
Degenerate Modes: 2 & 3/5 & 6
"Bending" Vibrations:1,2,3
"Bond Stretch" Vibrations:4,5,6
Highly Symmetric Mode:4
"Umbrella" Mode:1
Number of Bands Expected in an Experimental Spectrum of Gaseous Ammonia: 2 - because the 'infrared' value is represented on the y-axis of the infrared spectrum, and since two of the four possible bands produced (since we have four distinct IR values) have very small values (0.2711 and 1.06080), they are barely visible on the IR spectrum.
Charge Analysis
Charge of H: 0.375
Charge of N:-1.125
For nitrogen, you would expect a partial negative charge, because it is the third most electronegative element in the periodic table, paired with hydrogen which is electropositive. Therefore, the nitrogen molecule 'withdraws' electron density in each of the N-H bonds towards itself, making nitrogen delta negative, and hydrogen partial positive.
N2 Molecule
General Information
A summary of general information obtained from the results of the optimisation of the N2 molecule in the "Gaussian" software package is included below.
Calculation Method: RB3LYP
Basis Set:6-31G(d,p)
Final Energy, E(RB3LYP): -109.52412868au
RMS Gradient Norm: 0.00000010au
Point Group: D∞H
Optimised NN Bond Distance: 1.10550Å
Literature NN Bond Distance: 1.0976 Å[1] Thus, the computationally modelled bond length has a 0.72% percentage uncertainty, which is a very high accuracy, indicating that the "Gaussian" optimisation of N2 produces an excellent approximation to the actual physical molecule.
(Optimised) NN Bond Angle: N/A - 3 points are necessary to define an angle, and the molecule is diatomic, so there are only 2 points. The molecule is linear.
- ↑ DeKock R, Gray H. Chemical structure and bonding. Menlo Park, Calif.: Benjamin/Cummings Pub. Co.; 1980.
Item Table
The table below is found within the 'real' results text file produced from the optimisation of the N2 in the "Gaussian" software package. It is included in this report as proof that this optimisation reached completion.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000000 | 0.000450 | YES |
| RMS Force | 0.000000 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000000 | 0.001200 | YES |
Predicted change in Energy=-9.497820D-15 Optimization completed. Stationary point found.
JMol Image
Included below is a Jmol rotatable image of the optimised N2 molecule, generated in the "Gaussian" software.
N2 Molecule |
The optimisation file is linked here
Vibrational Analysis
Using the optimised N2 molecule, an analysis of the vibrational modes was performed in the "Gaussian" software, to determine the frequency at which distinct bond stretches/bends are produced, and the corresponding value (i.e. intensity of absorption of IR radiation) they would produce in a IR spectrum of the molecule. Included below is the frequency at which the N2 bond would vibrate when subjected to IR radiation, however, because the N2 molecule is completely symmetrical, this "bond stretching" vibration does not cause a change in dipole moment, and hence is IR "inactive", i.e. it does not prodcue a band in the IR spectrum, which is shown by the value of 0.0000 in the IR column (below).
The frequency value given in the above table is not negative, indicating that the optimisation has been successful.
Number Of Modes Expected:1
Degenerate Modes: N/A
"Bending" Vibrations: N/A
"Bond Stretch" Vibrations: 1
Highly Symmetric Mode: 1
"Umbrella" Mode: N/A
Number of Bands Expected in an Experimental Spectrum of Gaseous N2: 0 - because the 'infrared' value is represented on the y-axis of the infrared spectrum, and since this is 0.000, there is no peak produced, so no band is produced.
Charge Analysis
As expected, both nitrogen atoms have a charge of 0.0000, since they are identical atoms, so have and electronegativity difference of 0, i.e. the N2 bond is perfectly covalent, so neither atom withdraws electrons more than the other.
H2 Molecule
General Information
A summary of general information obtained from the results of the optimisation of the H2 molecule in the "Gaussian" software package is included below.
Calculation Method: RB3LYP
Basis Set:6-31G(d,p)
Final Energy, E(RB3LYP): -1.17853936a.u.
RMS Gradient Norm: 0.00002276a.u.
Point Group: D∞H
Optimised HH Bond Distance: 0.74274Å
Literature HH Bond Distance: 0.82±0.01Å[1] Thus, the computationally modelled bond length has a 9.42% produce uncertainty, which is reasonably accurate, indicating that the "Gaussian" optimisation of H2 produces a good approximation to the actual physical molecule. However, this percentage uncertainty is much higher than the percentage uncertainties associated to the optimised bond lengths of the other molecules (NH3, H2 and ClF). This could be related to the magnitude of the H-H bond- it has the smallest bond length of all of the included molecules, and the computational simulation may not have a fine enough precision.
(Optimised) HH Bond Angle: N/A - 3 points are necessary to define an angle, and the molecule is diatomic, so there are only 2 points. The molecule is linear.
Item Table
The table below is found within the 'real' results text file produced from the optimisation of the H2 in the "Gaussian" software package. It is included in this report as proof that this optimisation reached completion.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000039 | 0.000450 | YES |
| RMS Force | 0.000039 | 0.000300 | YES |
| Maximum Displacement | 0.000052 | 0.001800 | YES |
| RMS Displacement | 0.000073 | 0.001200 | YES |
Predicted change in Energy=-2.043043D-09 Optimization completed. Stationary point found.
JMol Image
Included below is a Jmol rotatable image of the optimised H2 molecule, generated in the "Gaussian" software.
H2 molecule |
The optimisation file is linked here
Vibrational Analysis
Using the optimised H2 molecule, an analysis of the vibrational modes was performed in the "Gaussian" software, to determine the frequency at which distinct bond stretches/bends are produced, and the corresponding value (i.e. intensity of absorption of IR radiation) they would produce in a IR spectrum of the molecule. Included below is the frequency at which the H2 bond would vibrate when subjected to IR radiation. However, in an analogous manner to the N2 molecule, because the H2 molecule is completely symmetrical, this "bond stretching" vibration does not cause a change in dipole moment, and hence is IR "inactive", i.e. it does not prodcue a band in the IR spectrum, which is shown by the value of 0.0000 in the IR column (below).
As is evident, the frequency given in the above table is not negative, indicating that the optimisation has been successful.
Number Of Modes Expected:1
Degenerate Modes: N/A
"Bending" Vibrations: N/A
"Bond Stretch" Vibrations: 1
Highly Symmetric Mode: 1
"Umbrella" Mode: N/A
Number of Bands Expected in an Experimental Spectrum of Gaseous N2: 0 - because the 'infrared' value is represented on the y-axis of the infrared spectrum, and since this is 0.000, there is no peak produced, so no band is produced.
Charge Analysis
As expected, both hydrogen atoms have a 0.000 charge, since they are identical atoms, so are equally electronegative, and hence, the shared pair of electrons are held exactly in the centre of the internuclear region, and hence, neither atoms has a greater/smaller partial charge.
Reaction Energies
Calculating the enthalpy of reaction for the Haber-Bosch process, i.e. the reaction: N2 + 3H2 -> 2NH3
E(NH3): -56.55776873au
2*E(NH3): -113.1155375au
E(N2): -109.52412868au
E(H2): -1.17853936au
3*E(H2): -3.53561808au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]: -0.05579074au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]: -146.48kJ/mol (2 decimal places)
Therefore, the ammonia product is more stable, since the forwards reaction (which produces ammonia) is exothermic, so energy has been 'released', i.e.we have gone from a higher energy state to a lower energy, more stable, state.
On comparison to a literature value, it can be observed that there is significant deviation between this computer simulated value (-146.48kJ/mol) and an experimental values (-92.22kJ/mol[1]). This is a large difference, giving a huge percentage uncertainty of 58.8%. This could be due to a number of reasons, for example, the computational approach uses several approximations which overlooks significant factors that influence the experimental value.
ClF Molecule
General Information
A summary of general information obtained from the results of the optimisation of the ClF molecule in the "Gaussian" software package is included below.
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy, E(RB3LYP): -559.94269589a.u.
RMS Gradient Norm: 0.00000436a.u.
Point Group: C∞V
Optimised ClF Bond Distance: 1.66390Å
Literature ClF Bond Distance: 1.63 Å[1] Thus, the computationally modelled bond length has a 2.1% percentage uncertainty, which is a high accuracy, showing that the "Gaussian" optimisation of ClF produces an good approximation to the actual physical molecule-this implies that all of the values calculated from this optimisation are reliable to use in further analysis.
Optimised Bond Angle: N/A - 3 points are necessary to define an angle, and the molecule is diatomic, so there are only 2 points. The molecule is linear.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedClF Bond Length
Item Table
The table below is found within the 'real' results text file produced from the optimisation of the ClF in the "Gaussian" software package. It is included in this report as proof that this optimisation reached completion.
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000008 | 0.000450 | YES |
| RMS Force | 0.000008 | 0.000300 | YES |
| Maximum Displacement | 0.000013 | 0.001800 | YES |
| RMS Displacement | 0.000019 | 0.001200 | YES |
Predicted change in Energy=-1.001268D-10 Optimization completed. Stationary point found.
JMol Image
Included below is a Jmol rotatable image of the optimised ClF molecule, generated in the "Gaussian" software.
ClF molecule |
The optimisation file is linked to here
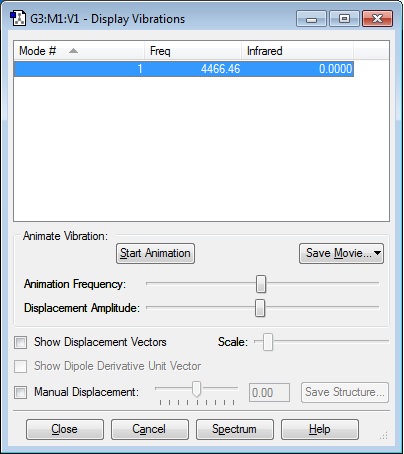
Vibrational Analysis
Using the optimised ClF molecule, an analysis of the vibrational modes was performed in the "Gaussian" software, to determine the frequency at which distinct bond stretches/bends are produced, and the corresponding value (i.e. intensity of absorption of IR radiation) they would produce in a IR spectrum of the molecule. Included below is the frequency at which the ClF bond would vibrate when subjected to IR radiation. The molecule is linear, and hence there is only one possible vibrational mode (a "bond stretch" vibration), however, because the ClF bond has a dipole moment (as chlorine and fluorine atoms have different electronegativities), this bond stretch produces a change in dipole moment, and hence, this vibration is IR active, and we will therefore observe one band in the IR spectrum of gaseous ClF.
As is evident, the frequency given in the above table is not negative, indicating that the optimisation has been successful.
Number Of Modes Expected:1
Degenerate Modes: N/A
"Bending" Vibrations: N/A
"Bond Stretch" Vibrations: 1
Highly Symmetric Mode: 1
"Umbrella" Mode: N/A
Number of Bands Expected in an Experimental Spectrum of Gaseous ClF: 1
Charge Analysis
Charge of Cl: +0.309
Charge of F: -0.309
As expected, fluorine, being the more electronegative element, hold the negative (partial) charge, since it more strongly draws the electrons towards itself, away from chlorine, which therefore obtains a partial negative charge.
Molecular Orbitals

This MO is occupied.It is the bonding sigma orbital (σ)composed of the chlorine 3s atomic orbital and the fluorine 2s atomic orbital. This MO is skewed towards the fluorine atom, because the fluorine atom is the more electronegative element in the ClF bond, and the more electronegative a given element, the greater its contribution to the bonding orbital. This MO has an overall favourable contribution to bonding, since all bonding orbitals are lower in energy than their constituent atomic orbitals, and hence, when filled with electrons, the overall energy of the ClF molecule is lowered (i.e. a stabilising effect). This MO is in the HOMO/LUMO region in terms of energy.
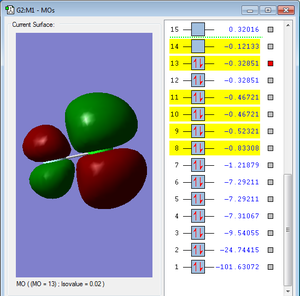
This MO is occupied, and it is the HOMO of the ClF molecule. This MO is a π* (antibonding) molecular orbital, composed of a chlorine 3p orbital and a fluorine 2p orbital. Chlorine has a greater contribution to the antibonding MO because chlorine is the less electronegative element, and hence contributes more to the antibonding molecular orbital. Since this is an occupied antibonding orbital, it reduces the bonding character of the ClF bond, making it weaker, since antibonding orbitals have a higher energy relative to their component atomic orbital, so the occupation of antibonding AOs raises the energy of the overall molecule.This MO is in the HOMO/LUMO region in terms of energy.
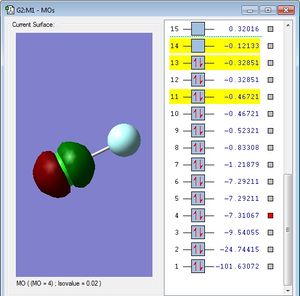
This MO is occupied. It is localised entirely on the chlorine atom. This is because it is composed of non-bonding chlorine 2p orbitals, therefore, this MO does not effect the bonding in ClF at all.The MO is fairly low in energy (-7.31067au), and cannot be considered as within the HOMO region, so is thought to be "deep" in energy. (It is too deep in energy to contribute to bonding).
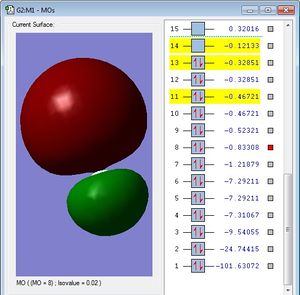
This MO is occupied,and is the antibonding sigma orbital(σ*)composed of the chlorine 3s atomic orbital, and the fluorine 2s atomic orbital. This MO is considered to be in the HOMO/LUMO region in terms of energy. Again, this MO is skewed towards chlorine, since it is an antibonding MO, and hence the more electropositive element (i.e. chlorine) contributes the most to this MO.Again, since this is an antibonding MO which is occupied with electrons, it serves to weaken the ClF bond by reducing the bonding character, and raising the energy of the ClF molecule, making it less stable.
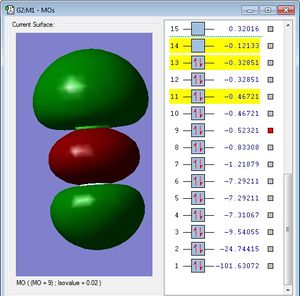
This MO is occupied. It is a bonding sigma orbital (σ)made up of a chlorine 3p atomic orbital, and a fluorine 2p atomic orbital. Again, it would be expected that the fluorine atomic orbital would have the greater contribution to the MO since it is more electronegative, however, the diagram is deceptive since the 2p fluorine atomic orbital is in fact considerably smaller than the chlorine 3p atomic orbital, so it appears that the chlorine 3p orbital has a greater contribution to this σ bonding orbital, but this is not, in fact, the case. This MO has an overall bonding effect on the ClF bond, because it increases the stability of the molecule when occupied with electrons, since all bonding orbitals have a lower energy than their constituent AOs, so when electrons accommodate them, the overall energy of the ClF molecule is reduced. This MO is therefore in the HOMO/LUMO energy region, since it contributes to the bonding nature of the ClF bond.