Rep:Mod:MMRLJ222526
Computational Lab - Introduction to Molecular Modelling 2
Ammonia (NH3) molecule ~
Using Gaussview 5.0, the optimisation of an ammonia molecule was calculated producing the following results...
Data Analysis:
- Name of Molecule: Ammonia (NH3)
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- Final Energy: -56.55776873 a.u.
- RMS Gradient: 0.00000485 a.u.
- Point Group: C3V
- N-H Bond Length: 1.01798 Å
- N-H Bond Angle: 105.74115°
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Image of Optimised Structure:
Optimised Ammonia Molecule |
Link to NH3 Optimisation log file:
Frequency Analysis:

- How many modes do you expect from the 3N-6 rule? 6 modes
- Which modes are degenerate (ie have the same energy)? Modes 2&3 and Modes 5&6
- Which modes are "bending" vibrations and which are "bond stretch" vibrations? Modes 1, 2 and 3 are "bending" vibrations. And modes 4, 5 and 6 are "bond stretching" modes.
- Which mode is highly symmetric? Mode 4
- One mode is known as the "umbrella" mode, which one is this? Mode 1
- How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2 bands. Modes 4,5 and 6 are unlikely to appear on the spectrum (have a low intensity peak) as the vibrations result only in a small change in dipole moment. And as modes 2 and 3 will contribute to the same peak, there will only be two peaks visible on an IR spectrum (due to type 1 and types 2&3 of vibrations in an Ammonia molecule).
Charge Analysis:
- Charge on the Nitrogen atom: -1.125
- Charge on the Hydrogen atom(s): +0.375
The results agree with my expectations. I would expect Nitrogen to have a negative charge as it is a highly electronegative atom around which the negatively charged electrons would cluster. Hydrogen on the other hand, I expected to have a positive charge as it is not an electronegative atom, therefore the electrons in the N-H bonds are drawn towards the Nitrogen atom leaving the Hydrogen with a positive charge.
Nitrogen (N2) molecule ~
Using Gaussview 5.0, the optimisation of a nitrogen molecule was calculated producing the following results...
Data Analysis:
- Name of Molecule: Nitrogen (N2)
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- Final Energy: -109.52412868 a.u.
- RMS Gradient: 0.00000060 a.u.
- Point Group: D∞h
- N≡N Bond Length: 1.10550 Å
- N≡N Bond Angle: 180°
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Image of Optimised Structure:
Optimised Nitrogen Molecule |
Link to N2 Optimisation log file:
Frequency Analysis:
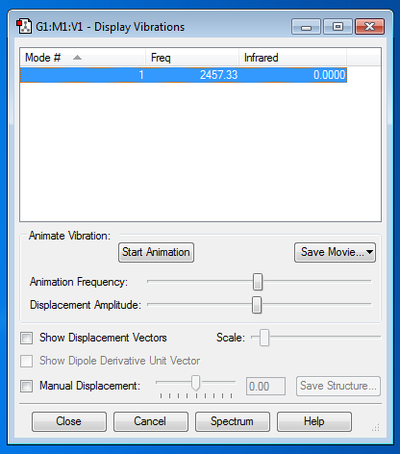
There are no negative frequencies, hence the optimisation has been completed.
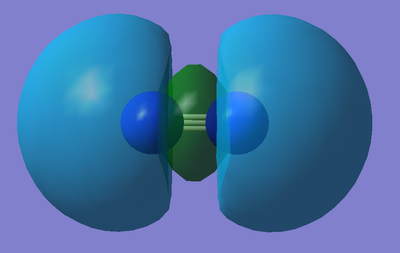
Molecular Orbitals of an N2 molecule:
Below are a few examples of occupied molecular orbitals involved in a molecule of Nitrogen...


Hydrogen (H2) molecule ~
Using Gaussview 5.0, the optimisation of a hydrogen molecule was calculated producing the following results...
Data Analysis:
- Name of Molecule: Hydrogen (H2)
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- Final Energy: -1.17853936 a.u.
- RMS Gradient: 0.00000017 a.u.
- Point Group: D∞h
- H-H Bond Length: 0.74279 Å
- H-H Bond Angle: 180°
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Image of Optimised Structure:
Optimised Hydrogen Molecule |
Link to H2 Optimisation log file:
Frequency Analysis:
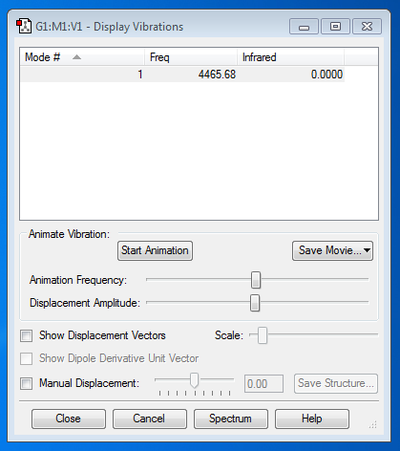
There are no negative frequencies, hence the optimisation has been completed.
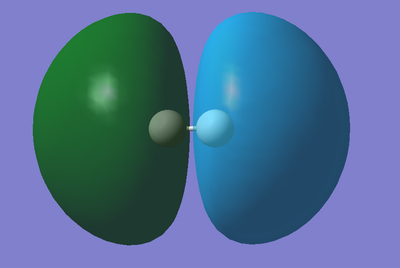
Molecular Orbitals of an H2 molecule:
Below are a couple of examples of molecular orbitals involved in a molecule of Hydrogen...

Energy Calculations ~
For the reaction: N2 + 3H2 -> 2NH3, the energy calculations are shown below...
- E(NH3)= -56.55776873 a.u.
- 2*E(NH3)= -113.1155375 a.u.
- E(N2)= -109.52412868 a.u.
- E(H2)= -1.17853936 a.u.
- 3*E(H2)= -3.53561808 a.u.
- ΔE=2*E(NH3)-[E(N2)+ 3*E(H2)]= -0.05579074 a.u.
In kJ/mol...
- ΔE=-146.4785879 kJ/mol
The negative change in energy suggests that the Ammonia gas is the most stable in the reaction as energy must be supplied in order to convert it back into the reactants, Nitrogen and Hydrogen.
Investigating the properties of a Water (H2O) molecule ~
Using Gaussview 5.0, the optimisation of a water molecule was calculated producing the following results...
Data Analysis:
- Name of Molecule: Water (H2O)
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d.p)
- Final Energy: -76.41973740 a.u.
- RMS Gradient: 0.00006276 a.u.
- Point Group: C2V
- O-H Bond Length: 0.96522 Å
- O-H Bond Angle: 103.74544°
Item Value Threshold Converged?
Maximum Force 0.000099 0.000450 YES
RMS Force 0.000081 0.000300 YES
Maximum Displacement 0.000128 0.001800 YES
RMS Displacement 0.000120 0.001200 YES
Image of Optimised Structure:
Optimised Water Molecule |
Link to H2O Optimisation log file:
Frequency Analysis:
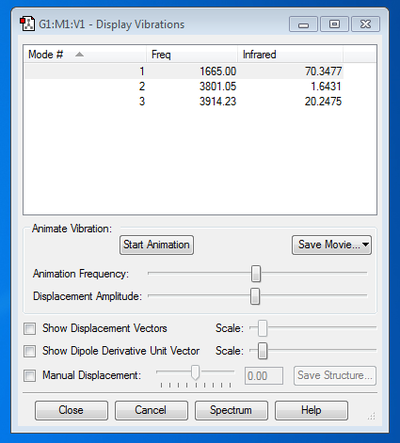
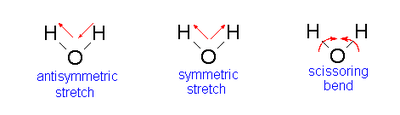
The results of the frequency analysis show that there are three vibrational modes occurring in a molecule of water (this agrees with the findings of previous experiments into this topic [1]). There are two bond stretching modes (a symmetric stretch corresponding to the frequency of 3801.05 Hz and an anti-symmetric stretch with the frequency of 3914.23 Hz) and one bending mode (also known as a scissoring action) with the lowest frequency of all, 1665.00 Hz. The absolute values for the vibrational frequencies differ slightly from literature results [1] however the large difference in frequency and energy between the stretching and bending modes can be seen in both sets of data. The diagram below depicts the different vibrational modes [2] ...
These results also imply that there will be only two bands visible on the IR spectrum of water. This is because the intensity of the symmetric stretch is close to zero as the vibration only causes a slight change in dipole moment. These predictions were confirmed through the generation of the IR spectrum below...

The largest peak is due to the bending mode therefore meaning the slightly less intense peak is as a result of the anti-symmetric stretch.
Charge Analysis:
- Charge on the Oxygen atom: -0.944
- Charge on the Hydrogen atom(s): +0.472
The results agree with my expectations. I would expect Oxygen to have a negative charge as it is a highly electronegative atom around which the negatively charged electrons would cluster. Hydrogen on the other hand, I expected to have a positive charge as it is not an electronegative atom, therefore the electrons in the O-H bonds are drawn towards the Oxygen atom leaving the Hydrogen with a positive charge.
Molecular Orbitals of an H2O molecule:
There are several molecular orbitals involved in the chemical bonding within a water molecule. The formation of such MO's can be expressed in a Molecular Orbital diagram such as the one below [3]...
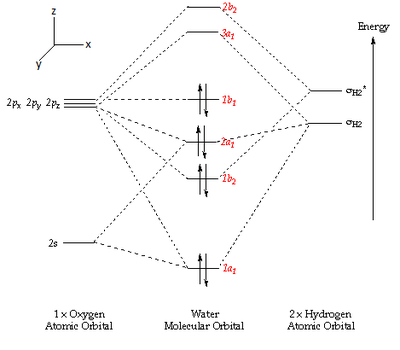
Five of the molecular orbitals involved are depicted below with corresponding descriptions [4],[5],[6]...
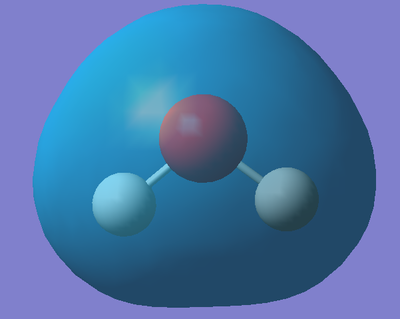
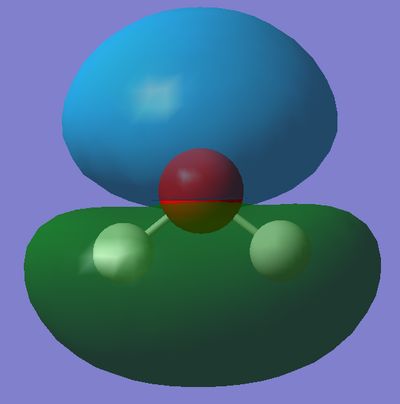
This is the molecular orbital that corresponds to 1a1 on the above diagram. This molecular orbital is formed from the overlap of the 2s orbital from Oxygen and 1s orbital(s) from the Hydrogen(s) and is fully occupied with two electrons. It is a bonding MO meaning the orbitals must have combined in phase. Furthermore, the orbital is relatively deep in energy (it's absolute energy value is -99736 a.u.) suggesting there is a large overlap of the orbitals. The majority of the contribution to this bonding MO comes from 2s orbital of the electronegative Oxygen atom, hence why the MO is rather spherical in shape.
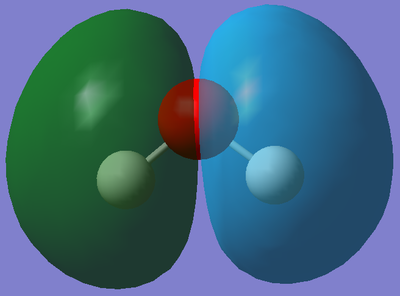
This is the molecular orbital that corresponds to 1b2 on the above diagram. This molecular orbital is formed from the overlap of the 2px orbital from Oxygen and 1s orbital(s) from the Hydrogen(s) and is fully occupied with two electrons. It is a bonding MO meaning the orbitals must have combined in phase. Furthermore, as the orbital is stabilised, it means it is deep in energy (it's absolute energy value is -0.51503 a.u.) suggesting there is a large overlap between the orbitals; however as it has a greater energy than the MO depicted above, it means the overlap of these orbitals isn't as large.
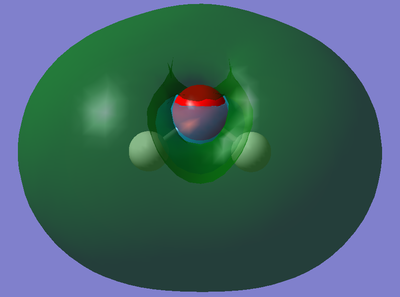
This is the molecular orbital that corresponds to 2a1 on the above diagram. This molecular orbital is formed from mixing of an MO orbital (formed from the overlap of the 2s orbital from Oxygen and 1s orbital(s) from the Hydrogen(s)) and the 2pz orbital from Oxygen. It is fully occupied with two electrons and is a bonding MO meaning the orbitals must have combined in phase. Furthermore, the orbital is deep in energy (it's absolute energy value is -0.37102 a.u.) because the mixing stabilises the molecular orbital more therefore reducing its energy to lower than that of the MO described below.
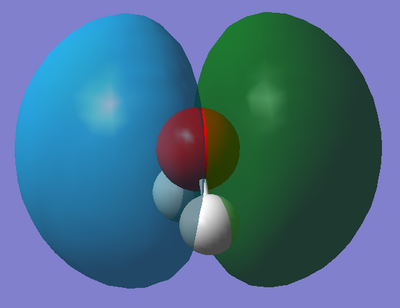
This is the molecular orbital that corresponds to 1b1 on the above diagram. This molecular orbital is formed from the unchanged (non-bonding) 2py orbital from Oxygen and is fully occupied with two electrons. Furthermore, this orbital has an absolute energy value of -0.29197 a.u. which is higher than that of the MO formed by mixing suggesting that the unchanged 2py orbital is less stable. This p-orbital contributes to a non-bonding MO because there is no other orbitals with the same symmetry for it to interact and overlap with. Moreover, this orbital can be described as the HOMO - (Highest Occupied Molecular Orbital).
This is the molecular orbital that corresponds to 3a1 on the above diagram. This molecular orbital is formed from the overlap of the 2s orbital from Oxygen and 1s orbital(s) from the Hydrogen(s) and is unoccupied with zero electrons. It is an anti-bonding MO meaning the orbitals must have combined out of phase (i.e. in anti-phase). Furthermore, this orbital has the highest energy of the 5 orbitals studied here (+0.06538 a.u.); this is due to the fact that anti-bonding molecular orbitals are destabilised when formed hence increasing their energy. It has also been established that anti-bonding MOs are always de-stabilised more than bonding MOs are stabilised. And so because this molecular orbital is the first unoccupied MO, it can be described as the LUMO (Lowest Unoccupied Molecular Orbital). Finally, the largest contribution to this anti-bonding orbital will come from the least electronegative Hydrogen atom(s).
On a final note, the molecular orbital with the lowest energy (-19.13799 a.u.) is that formed from the 1s orbital of the Oxygen atom. It is not included on the MO diagram because the electrons which occupy this orbital are not valence electrons and therefore do not contribute to the bonding of the molecule. Furthermore, all the bonding molecular orbitals relate to the formation of sigma bonds.
References ~
<references> [1] [2] [3] [4] [5] [6]
- ↑ 1.0 1.1 1.2 Benedict, W. S., Guilar, N., and Plyer E. K., 1956. J Chem. Phys. 24, pp. 1139
- ↑ 2.0 2.1 https://chemistry.boisestate.edu/richardbanks/inorganic/electromagnetic%20spectrum/vibrational_modes.htm
- ↑ 3.0 3.1 http://www.ch.ic.ac.uk/vchemlib/course/mo_theory/
- ↑ 4.0 4.1 http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_15/node3.html
- ↑ 5.0 5.1 Autschback, J., 2012. Orbitals: Some Fiction and Some Facts. J. Chem. Educ., 89 (8), pp. 1032–1040
- ↑ 6.0 6.1 http://www1.lsbu.ac.uk/water/h2o_orbitals.html
